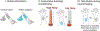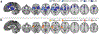A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions - PubMed (original) (raw)
Meta-Analysis
A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions
Lauren Y Atlas et al. Handb Exp Pharmacol. 2014.
Abstract
Placebo treatments reliably reduce pain in the clinic and in the lab. Because pain is a subjective experience, it has been difficult to determine whether placebo analgesia is clinically relevant. Neuroimaging studies of placebo analgesia provide objective evidence of placebo-induced changes in brain processing and allow researchers to isolate the mechanisms underlying placebo-based pain reduction. We conducted formal meta-analyses of 25 neuroimaging studies of placebo analgesia and expectancy-based pain modulation. Results revealed that placebo effects and expectations for reduced pain elicit reliable reductions in activation during noxious stimulation in regions often associated with pain processing, including the dorsal anterior cingulate, thalamus, and insula. In addition, we observed consistent reductions during painful stimulation in the amygdala and striatum, regions implicated widely in studies of affect and valuation. This suggests that placebo effects are strongest on brain regions traditionally associated with not only pain, but also emotion and value more generally. Other brain regions showed reliable increases in activation with expectations for reduced pain. These included the prefrontal cortex (including dorsolateral, ventromedial, and orbitofrontal cortices), the midbrain surrounding the periaqueductal gray, and the rostral anterior cingulate. We discuss implications of these findings as well as how future studies can expand our understanding of the precise functional contributions of the brain systems identified here.
Figures
Fig. 1
Typical neuroimaging placebo paradigm. In a typical placebo study, participants are given an inert treatment (e.g., a topical cream) along with verbal instructions (e.g., “This is a potent analgesic”) that induce expectations for pain relief. This is compared to a control condition—the same inert substance without expected pain relief. To reinforce verbal instructions, the placebo is paired with reduced stimulus intensity during an associative learning, or conditioning, phase. Finally, participants go through neuroimaging testing during a test phase during which the same stimuli are administered under both control and placebo conditions and experimenters test whether pain reports and brain responses are modulated by beliefs about treatment
Fig. 2
Meta-analysis 1: expectancy-based pain modulation. (a) Peaks included in a meta-analysis of expectancy-based reductions during pain. (b) Brain regions that showed reliable reductions during placebo administration and expectations for reduced pain (see Table 3). (c) Peaks included in meta-analysis of modulatory increases during pain. (d) Regions that showed consistent increases during anticipation or pain stimulation with expectations for reduced pain (see Table 4)
Fig. 3
Decreases: treatment expectancies vs. stimulus expectancies. (a) Brain regions that showed reliable reductions during placebo analgesia (see Table 5). (b) Differences between placebo analgesia and stimulus expectancy-induced reductions (placebo analgesia > stimulus expectancies; see Table 8). Left anterior insula was significantly more likely to show reductions with placebo analgesia than with stimulus expectancies
Fig. 4
Modulatory increases: treatment expectancies vs. stimulus expectancies. (a) Brain regions that showed reliable increases prior to or during placebo analgesia (see Table 6). (b) Brain regions that showed reliable increases as a function of stimulus expectancy (i.e., increased activity with expectation for reduced pain; see Table 7). (c) Differences between placebo analgesia and stimulus expectancy-induced increases (placebo analgesia > stimulus expectancies; see Table 8). (d) Regions that showed larger increases with stimulus expectancy than placebo analgesia (see Table 9)
Similar articles
- Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia.
Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Zeidan F, et al. J Neurosci. 2015 Nov 18;35(46):15307-25. doi: 10.1523/JNEUROSCI.2542-15.2015. J Neurosci. 2015. PMID: 26586819 Free PMC article. Clinical Trial. - Neuroimaging study of placebo analgesia in humans.
Qiu YH, Wu XY, Xu H, Sackett D. Qiu YH, et al. Neurosci Bull. 2009 Oct;25(5):277-82. doi: 10.1007/s12264-009-0907-2. Neurosci Bull. 2009. PMID: 19784082 Free PMC article. Review. - Placebo improves pleasure and pain through opposite modulation of sensory processing.
Ellingsen DM, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S. Ellingsen DM, et al. Proc Natl Acad Sci U S A. 2013 Oct 29;110(44):17993-8. doi: 10.1073/pnas.1305050110. Epub 2013 Oct 14. Proc Natl Acad Sci U S A. 2013. PMID: 24127578 Free PMC article. Clinical Trial. - How expectations shape pain.
Atlas LY, Wager TD. Atlas LY, et al. Neurosci Lett. 2012 Jun 29;520(2):140-8. doi: 10.1016/j.neulet.2012.03.039. Epub 2012 Mar 21. Neurosci Lett. 2012. PMID: 22465136 Review. - Brain mechanisms of social touch-induced analgesia in females.
López-Solà M, Geuter S, Koban L, Coan JA, Wager TD. López-Solà M, et al. Pain. 2019 Sep;160(9):2072-2085. doi: 10.1097/j.pain.0000000000001599. Pain. 2019. PMID: 31241496
Cited by
- Effects of Positive and Negative Expectations on Human Pain Perception Engage Separate But Interrelated and Dependently Regulated Cerebral Mechanisms.
Shih YW, Tsai HY, Lin FS, Lin YH, Chiang CY, Lu ZL, Tseng MT. Shih YW, et al. J Neurosci. 2019 Feb 13;39(7):1261-1274. doi: 10.1523/JNEUROSCI.2154-18.2018. Epub 2018 Dec 14. J Neurosci. 2019. PMID: 30552181 Free PMC article. - Feelings of Clinician-Patient Similarity and Trust Influence Pain: Evidence From Simulated Clinical Interactions.
Losin EAR, Anderson SR, Wager TD. Losin EAR, et al. J Pain. 2017 Jul;18(7):787-799. doi: 10.1016/j.jpain.2017.02.428. Epub 2017 May 4. J Pain. 2017. PMID: 28479279 Free PMC article. - The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology.
Hiser J, Koenigs M. Hiser J, et al. Biol Psychiatry. 2018 Apr 15;83(8):638-647. doi: 10.1016/j.biopsych.2017.10.030. Epub 2017 Nov 20. Biol Psychiatry. 2018. PMID: 29275839 Free PMC article. Review. - Instructions and experiential learning have similar impacts on pain and pain-related brain responses but produce dissociations in value-based reversal learning.
Atlas LY, Dildine TC, Palacios-Barrios EE, Yu Q, Reynolds RC, Banker LA, Grant SS, Pine DS. Atlas LY, et al. Elife. 2022 Nov 1;11:e73353. doi: 10.7554/eLife.73353. Elife. 2022. PMID: 36317867 Free PMC article. - Analgesia Effect of Verum and Sham Acupuncture Treatments in Primary Dysmenorrhea: A MRI Pilot Study.
Peng SL, Yang HC, Lee YC, Chen CM, Chen YY, Tu CH. Peng SL, et al. J Pers Med. 2021 Nov 23;11(12):1244. doi: 10.3390/jpm11121244. J Pers Med. 2021. PMID: 34945716 Free PMC article.
References
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain (Lond) 9:463–484 - PubMed
- Atlas LY, Wager TD (2012) How expectations shape pain. Neurosci Lett 520:140–148 - PubMed
- Atlas LY, Wager TD (2013) Expectancies and beliefs: insights from cognitive neuroscience In: Ochsner KN, Kosslyn SM (eds) Oxford handbook of cognitive neuroscience. Oxford University Press, Oxford, NY, pp 359–381
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous