Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications - PubMed (original) (raw)
. 2014 Nov;124(11):4941-52.
doi: 10.1172/JCI76864. Epub 2014 Oct 20.
Anshita Rai, Neeraja Kambham, Joyce F Sung, Nirbhai Singh, Matthew Petitt, Sabita Dhal, Rani Agrawal, Richard E Sutton, Maurice L Druzin, Sanjiv S Gambhir, Balamurali K Ambati, James C Cross, Nihar R Nayak
- PMID: 25329693
- PMCID: PMC4347223
- DOI: 10.1172/JCI76864
Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications
Xiujun Fan et al. J Clin Invest. 2014 Nov.
Abstract
There is strong evidence that overproduction of soluble fms-like tyrosine kinase-1 (sFLT1) in the placenta is a major cause of vascular dysfunction in preeclampsia through sFLT1-dependent antagonism of VEGF. However, the cause of placental sFLT1 upregulation is not known. Here we demonstrated that in women with preeclampsia, sFLT1 is upregulated in placental trophoblasts, while VEGF is upregulated in adjacent maternal decidual cells. In response to VEGF, expression of sFlt1 mRNA, but not full-length Flt1 mRNA, increased in cultured murine trophoblast stem cells. We developed a method for transgene expression specifically in mouse endometrium and found that endometrial-specific VEGF overexpression induced placental sFLT1 production and elevated sFLT1 levels in maternal serum. This led to pregnancy losses, placental vascular defects, and preeclampsia-like symptoms, including hypertension, proteinuria, and glomerular endotheliosis in the mother. Knockdown of placental sFlt1 with a trophoblast-specific transgene caused placental vascular changes that were consistent with excess VEGF activity. Moreover, sFlt1 knockdown in VEGF-overexpressing animals enhanced symptoms produced by VEGF overexpression alone. These findings indicate that sFLT1 plays an essential role in maintaining vascular integrity in the placenta by sequestering excess maternal VEGF and suggest that a local increase in VEGF can trigger placental overexpression of sFLT1, potentially contributing to the development of preeclampsia and other pregnancy complications.
Figures
Figure 8. Effects of placental sFlt1 knockdown with or without endometrial VEGF overexpression.
(A–L) Placental sFlt1 knockdown. Upon placenta-specific sFLT1 shRNA expression, widespread hemorrhaging in the fetus (B) and at the placental-decidual junction (D) was observed on GD18 compared with controls (A and C). Histological examination of sFLT1 shRNA–expressing placentas revealed extraordinary dilation of some maternal blood sinuses (arrowheads) in the labyrinth (E and F) and fibrin deposition (arrow) in these spaces (G and H). (I and J) MSB staining revealed extravasated fibrin (arrow) in adjacent areas. Placental sFlt1 knockdown did not affect implantation rate (K), whereas the fetal resorption rate significantly increased (L). (M–V) Placental sFlt1 knockdown enhanced the deleterious effects in Endo-VEGF animals. Pregnancies surviving to GD16 exhibited (M) excessive vaginal bleeding and (N–P) termination of pregnancy or resorption (arrows denote resorption sites) as well as (Q) widespread and extensive hemorrhaging in fetuses and placentas (arrowheads) and in deciduas at the maternal-fetal junction (asterisk). Histological examination (R–T) revealed widespread dilation and congestion of maternal blood sinuses (arrowheads) in the labyrinth, venous sinuses, and veins at maternal-fetal junctions, and MSB staining (U and V) demonstrated extensive fibrin extravasation (arrows) in the labyrinth and at the maternal-fetal junction. Results are mean ± SD. *P < 0.05 (n = 15). Scale bars: 2 mm (A–D); 500 μm (E, F, and R); 50 μm (G–J); 100 μm (S–V).
Figure 7. Effective sFlt1 knockdown in placentas by trophoblast-specific sFLT1 shRNA expression.
(A and B) Blastocysts transduced with lentiviruses expressing GFP control (A) or both sFLT1 shRNA and GFP (B) expressed GFP (denoted by #) in the trophectoderm, but not the inner cell mass. (C and D) Full-thickness placenta on GD18 showing GFP expression in trophoblast lineages from blastocysts transduced with control (C) and sFLT1 shRNA (D) viruses. (E and F) Magnified images from C and D showing GFP expression in the labyrinth layer. (G and H) ISH showing dramatic reduction of sFlt1 signal in sFLT1 shRNA–expressing placentas (H) relative to controls (G); signals were found primarily in GlyTCs and spongiotrophoblasts in the JZ (arrows) and the invading GlyTCs below the giant cell layer (arrowheads). Ch, chorionic plate; La, labyrinth layer. (I) Representative Western blots showing a substantial sFLT1 shRNA–mediated decrease in placental sFLT1 levels on GD18 without changes in VEGF levels. (J–M) qPCR of placental sFlt1 mRNA (J), quantification of placental sFLT1 protein by Western blot (K), and ELISA of maternal serum sFLT1 protein (M) revealed significant reductions at GD18 in placental sFlt1 knockdown animals, whereas (L) placental VEGF/sFLT1 protein ratio was significantly increased by sFlt1 knockdown. Results are mean ± SD. *P < 0.05 (n = 5). Scale bars: 20 μm (A and B); 500 μm (C, D, G, and H); 100 μm (E and F).
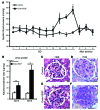
Figure 6. Endometrial VEGF overexpression leads to preeclampsia-like symptoms in mice.
(A) Mean systolic blood pressure from control and Endo-VEGF animals with >4 live fetuses at term on different days of pregnancy. Endo-VEGF animals showed significant increases in blood pressure beginning at GD14, and blood pressure dropped to normal levels after delivery. (B) Significantly elevated albumin/creatinine ratio at both GD15 and GD18 in Endo-VEGF mice. (C–F) Staining of kidney sections with H&E (C and E) and PAS (D and F) revealed focal increase in glomerular cellularity and cytoplasmic swelling (endotheliosis) in Endo-VEGF animals. Results are mean ± SD. *P < 0.05 (n = 5). Scale bars: 20 μm (C–F).
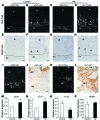
Figure 5. Effects of endometrial VEGF overexpression on placentas of surviving pregnancies that did not exhibit severe vascular changes.
(A–H) Trophoblast-lined SpAs at the JZ (A–D) and endothelium-lined SpAs in deeper decidua, 300 μm below the JZ (E–H). There were no marked changes between control and Endo-VEGF animals in either trophoblast-invaded (CK8-positive; arrowheads) SpAs (B and D) or endothelium-lined SpAs (F and H). (I–L) Expansion of the JZ, specifically GlyTCs (arrowheads), and dilation of venous channels (arrows) in Endo-VEGF animals (K and L) compared with controls (I and J). (M–P) Upon VEGF overexpression, there were no changes in SpA and central canal diameter (M), but significant increases were observed in cross-sectional area and diameter of venous channels (N) and in total JZ and GlyTC areas (O). (P) The depth of trophoblast invasion into the decidua was not significantly changed between groups. Sp, spongiotrophoblast. Results are mean ± SD. *P < 0.05 (n = 15). Scale bars: 50 μm (A–H); 100 μm (I and K); 20 μm (J and L).
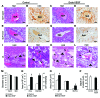
Figure 4. Overexpression of VEGF in the endometrium leads to increased pregnancy loss and widespread vascular deformity in the placenta, specifically in the venous channels and veins at the maternal-fetal interface.
(A–F) VEGF overexpression significantly increased resorption rate (A) and significantly decreased number of pups per litter (B), placental weight (C), and fetal weight (D–F) on GD18. (G and H) Vaginal bleeding occurred in most animals overexpressing VEGF, starting around GD10. (I and J) Gravid uteri in Endo-VEGF animals showed widespread hemorrhaging (arrowheads) compared with controls on GD12. (K–R) Histology of placental sections from control (K, M, O, and Q) and Endo-VEGF animals (L, N, P, and R), assessed by H&E (K–P) or martius scarlet blue (MSB) staining for extravasated fibrin (Q and R). Endo-VEGF animals showed severe dilation and congestion of venous sinuses and veins (white arrowheads) at the maternal-fetal junctions (L and N). Some of these venous sinuses exhibited focal, missing endothelial linings and hemorrhaging (black arrowheads) and extravasated fibrin (arrows) dissecting into the adjacent tissues (P and R). Results are mean ± SD. *P < 0.05 (n = 15). Scale bars: 10 mm (E and F); 500 μm (K and L); 100 μm (M and N); 20 μm (O–R).
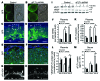
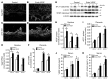
Figure 3. Endometrial VEGF overexpression leads to stimulation of placental sFLT1 production.
(A–F) Vegf and sFlt1 mRNA expression by ISH (A–D) on GD18 (sections in C and D adjacent to sections in A and B) and by qPCR on GD12, GD15, and GD18 in deciduas and placentas (E and F). Increases in the Vegf signal (arrows) in deciduas (B and E) and in the sFlt1 signal (arrowheads) in placentas (D and F) were evident in Endo-VEGF animals. (G–K) Similarly, significant increases in decidual VEGF protein expression were observed by Western blot of Endo-VEGF tissues (G and H). Corresponding increases in sFLT1 protein expression were found in the placenta (by Western blot; G and I) and in serum (by ELISA; K) of the same animals. No significant changes in circulating VEGF levels (bound plus free) were found by ELISA (J). Results are mean ± SD. *P < 0.05 (n = 5). Scale bars: 200 μm (A–D).
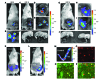
Figure 2. Development of an endometrium-specific gene delivery method: persistent transgene expression throughout pregnancy after LV-Fluc/GFP transfection of endometrial stromal cells.
(A–F) First pregnancy. Bioluminescence imaging of uteri during laparotomy on GD8 (A and B) and GD19 (C–F). There was a marked increase in Fluc signal at the implantation sites on GD8 (A and B) that was maintained through GD19 (C and D). The signal persisted in the uterine wall (E) after delivery of the fetuses and placentas (F) by cesarean section. (G–J) Second pregnancy, following mating 30 days after first delivery. As in the first pregnancy, the signal increased during pregnancy (G–I) and persisted in the uterine wall (I) after removal of the fetuses and placentas (J) by cesarean section on GD19. (K–P) Artificial decidualization. (K) Live bioluminescence imaging before induction of artificial decidualization (AD) in LV-Fluc/GFP–transfected uterus (both horns). (L) Bioluminescence imaging 3 days after artificial decidualization induction in 1 horn. Note the dramatic increase in Fluc signal in the decidualized horn (M). (N–P) Colocalization (P) of GFP (N) and BrdU (O) to decidual cells; numerous GFP-expressing cells were BrdU positive, which suggests that the increase in Fluc signal during pregnancy may have resulted from proliferation of LV-Fluc/GFP–transfected endometrial cells. Scale bar: 20 μm (N–P).
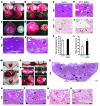
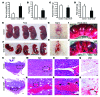
Figure 1. Increased VEGF expression in decidual cells and sFLT1 expression in adjacent EVTs in preeclamptic women.
(A–H) VEGF and sFLT1 mRNA expression by ISH (basal plate). Dashed lines demarcate the border between decidua (De) and placenta. Vi, villi. In specimens from both control and preeclamptic (PE) women, VEGF was expressed specifically in maternal decidual cells (A, E, C, and G), and sFLT1 in adjacent EVTs (arrowheads; B, F, D, and H), but levels of both were dramatically elevated in preeclamptic specimens. (I–L) VEGF (I) and sFLT1 (K) ISH and CK7 IHC (J and L; sections adjacent to I and K). (M–P) VEGF (M), sFLT1 (N), PLGF (O), and VEGFB (P) total mRNA levels in placental basal plate samples from control and preeclamptic women, determined by qPCR. In preeclampsia, while levels of both VEGF and sFLT1 significantly increased, PLGF significantly decreased, and VEGFB remained unchanged. Results are mean ± SD. *P < 0.05 (n = 15). Scale bars: 100 μm.
Comment in
- sFLT1 in preeclampsia: trophoblast defense against a decidual VEGFA barrage?
Adamson SL. Adamson SL. J Clin Invest. 2014 Nov;124(11):4690-2. doi: 10.1172/JCI78532. Epub 2014 Oct 20. J Clin Invest. 2014. PMID: 25329689 Free PMC article.
Similar articles
- Trophoblast expression of fms-like tyrosine kinase 1 is not required for the establishment of the maternal-fetal interface in the mouse placenta.
Hirashima M, Lu Y, Byers L, Rossant J. Hirashima M, et al. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15637-42. doi: 10.1073/pnas.2635424100. Epub 2003 Dec 10. Proc Natl Acad Sci U S A. 2003. PMID: 14668430 Free PMC article. - VEGF Maintains Maternal Vascular Space Homeostasis in the Mouse Placenta through Modulation of Trophoblast Giant Cell Functions.
Fan X, Muruganandan S, Shallie PD, Dhal S, Petitt M, Nayak NR. Fan X, et al. Biomolecules. 2021 Jul 20;11(7):1062. doi: 10.3390/biom11071062. Biomolecules. 2021. PMID: 34356686 Free PMC article. - Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Maynard SE, et al. J Clin Invest. 2003 Mar;111(5):649-58. doi: 10.1172/JCI17189. J Clin Invest. 2003. PMID: 12618519 Free PMC article. - A possible placental factor for preeclampsia: sFlt-1.
Kita N, Mitsushita J. Kita N, et al. Curr Med Chem. 2008;15(7):711-5. doi: 10.2174/092986708783885309. Curr Med Chem. 2008. PMID: 18336285 Review. - Placental-specific sFLT-1: role in pre-eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis.
Palmer KR, Tong S, Kaitu'u-Lino TJ. Palmer KR, et al. Mol Hum Reprod. 2017 Feb 10;23(2):69-78. doi: 10.1093/molehr/gaw077. Mol Hum Reprod. 2017. PMID: 27986932 Review.
Cited by
- microRNA-646 inhibits angiogenesis of endothelial progenitor cells in pre-eclamptic pregnancy by targeting the VEGF-A/HIF-1α axis.
Dong D, Khoong Y, Ko Y, Zhang Y. Dong D, et al. Exp Ther Med. 2020 Sep;20(3):1879-1888. doi: 10.3892/etm.2020.8929. Epub 2020 Jun 24. Exp Ther Med. 2020. PMID: 32782496 Free PMC article. - Induction of the PPARγ (Peroxisome Proliferator-Activated Receptor γ)-GCM1 (Glial Cell Missing 1) Syncytialization Axis Reduces sFLT1 (Soluble fms-Like Tyrosine Kinase 1) in the Preeclamptic Placenta.
Armistead B, Kadam L, Siegwald E, McCarthy FP, Kingdom JC, Kohan-Ghadr HR, Drewlo S. Armistead B, et al. Hypertension. 2021 Jul;78(1):230-240. doi: 10.1161/HYPERTENSIONAHA.121.17267. Epub 2021 May 24. Hypertension. 2021. PMID: 34024123 Free PMC article. - Preeclampsia: Maternal Systemic Vascular Disorder Caused by Generalized Endothelial Dysfunction Due to Placental Antiangiogenic Factors.
Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T. Tomimatsu T, et al. Int J Mol Sci. 2019 Aug 30;20(17):4246. doi: 10.3390/ijms20174246. Int J Mol Sci. 2019. PMID: 31480243 Free PMC article. Review. - The causal role of circulating immunity-inflammation in preeclampsia: A Mendelian randomization.
Xue X, Guo C, Fan C, Lei D. Xue X, et al. J Clin Hypertens (Greenwich). 2024 May;26(5):474-482. doi: 10.1111/jch.14775. Epub 2024 Mar 12. J Clin Hypertens (Greenwich). 2024. PMID: 38476059 Free PMC article. - Impacts of Gestational F-53B Exposure on Fetal Neurodevelopment: Insights from Placental and Thyroid Hormone Disruption.
Zhao S, Sun Y, Duan J, Zhang T, Xiao Y, Zhu Y, Jia Y, Zhong W, Zhu L. Zhao S, et al. Environ Health (Wash). 2024 Dec 11;3(3):308-320. doi: 10.1021/envhealth.4c00158. eCollection 2025 Mar 21. Environ Health (Wash). 2024. PMID: 40144320 Free PMC article.
References
- Zhou Y, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–1423. doi: 10.1016/S0002-9440(10)62567-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous