BRAF inhibitor-associated ERK activation drives development of chronic lymphocytic leukemia - PubMed (original) (raw)
Case Reports
. 2014 Nov;124(11):5074-84.
doi: 10.1172/JCI76539. Epub 2014 Oct 20.
Frank Meiss, Justin Mastroianni, Thorsten Zenz, Hana Andrlova, Nimitha R Mathew, Rainer Claus, Barbara Hutter, Stefan Fröhling, Benedikt Brors, Dietmar Pfeifer, Milena Pantic, Ingrid Bartsch, Timo S Spehl, Philipp T Meyer, Justus Duyster, Katja Zirlik, Tilman Brummer, Robert Zeiser
- PMID: 25329694
- PMCID: PMC4347247
- DOI: 10.1172/JCI76539
Case Reports
BRAF inhibitor-associated ERK activation drives development of chronic lymphocytic leukemia
Niuscha Yaktapour et al. J Clin Invest. 2014 Nov.
Abstract
Patients with BRAFV600E/K-driven melanoma respond to the BRAF inhibitor vemurafenib due to subsequent deactivation of the proliferative RAS/RAF/MEK/ERK pathway. In BRAF WT cells and those with mutations that activate or result in high levels of the BRAF activator RAS, BRAF inhibition can lead to ERK activation, resulting in tumorigenic transformation. We describe a patient with malignant melanoma who developed chronic lymphocytic leukemia (CLL) in the absence of RAS mutations during vemurafenib treatment. BRAF inhibition promoted patient CLL proliferation in culture and in murine xenografts and activated MEK/ERK in primary CLL cells from additional patients. BRAF inhibitor-driven ERK activity and CLL proliferation required B cell antigen receptor (BCR) activation, as inhibition of the BCR-proximal spleen tyrosine kinase (SYK) reversed ERK hyperactivation and proliferation of CLL cells from multiple patients, while inhibition of the BCR-distal Bruton tyrosine kinase had no effect. Additionally, the RAS-GTP/RAS ratio in primary CLL cells exposed to vemurafenib was reduced upon SYK inhibition. BRAF inhibition increased mortality and CLL expansion in mice harboring CLL xenografts; however, SYK or MEK inhibition prevented CLL proliferation and increased animal survival. Together, these results suggest that BRAF inhibitors promote B cell malignancies in the absence of obvious mutations in RAS or other receptor tyrosine kinases and provide a rationale for combined BRAF/MEK or BRAF/SYK inhibition.
Figures
Figure 6. Impact of SYK inhibition on BRAF inhibitor–induced primary CLL proliferation.
Primary CLL cells of different patients were exposed to DMSO or vemurafenib (1 μM), and the percentage of living cells relative to all cells (patients n = 7) when the MEK inhibitor (A) or the Syk inhibitor (B) was included, was determined for different time points. **P < 0.01; ***P < 0.001. (C) The amount of CLL cells in the peripheral blood of Rag2–/– γC–/– mice relative to start of treatment. * P < 0.05 when the group was compared with the vemurafenib-only group. (D) The survival of the Rag2–/– γC–/– mice treated with vemurafenib (24 mg/kg/d) alone or in combination with fostamatinib (60 mg/kg/d) or trametinib (1 mg/kg/every other day for 12 days) is shown (Vem vs. Vem/Fosta, P < 0.001; Vem vs. Vem/Tram, P < 0.001) (E) Proposed mechanism illustrating how BRAF inhibition could cooperate with SYK in paradoxical ERK activation. Vemurafenib binds to 1 protomer, e.g., BRAF, and stimulates a drug-free RAF molecule, e.g., RAF1, in an allosteric and RAS-dependent manner (yellow arrow). Active and RAF-binding competent RAS is supplied via SYK, which is hyperactivated due to the autonomous signaling capacity of the CLL-specific BCR.
Figure 5. Impact of BRAF inhibition on viability, proliferation, and in vivo expansion of primary CLL cells.
(A–C) Primary CLL cells from different patients were highly purified (>97% CD19+CD5+) and exposed to DMSO or vemurafenib. (A) The percentage of living cells relative to all cells (patients n = 3) or the absolute number of CLL cells (patients n = 4) (B) was determined for different time points. (C) Metabolic activity measured by MTT on day 52 of culture was higher in primary CLL cells (patients n = 3) when exposed to vemurafenib compared with DMSO. One of 3 independent experiments with similar results is shown. *P < 0.05; **P < 0.01. (D and E) On day 0, the amount of 2.5 × 107 patient-derived CD19+CD5+ cells was injected i.p. and i.v. (D) The amount of CLL cells in the peripheral blood of Rag2–/– γC–/– mice on days 0 and 7 relative to start of treatment. (E) The survival of the Rag2–/– γC–/– mice treated with DMSO or vemurafenib (24 mg/kg/d) is shown for the CLL cells derived from 3 different patients.
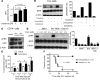
Figure 4. Impact of BRAF and SYK/MEK inhibition on ERK activation in primary CLL cells.
(A–F) Primary CLL cells from multiple patients were highly purified (>97% CD19+CD5+) and exposed to the indicated inhibitors. (A) Representative Western blot analysis for pERK and tERK of the protein lysate at the indicated concentrations of vemurafenib and dabrafenib for patients 1 and 2. The experiment was performed 2 times with similar results. (B) Quantification of the pERK/14-3-3 ratios and pERK/tERK ratios of 10 different patients are displayed (Vem, 1 μM). (C) Addition of the SYK inhibitor R406 decreased the ERK phosphorylation as shown for the protein lysates for patient 3. The experiment was performed 2 times with similar results. (D) Quantification of the pERK/14-3-3 ratios and pERK/tERK ratios of 9 and 5 different patients, respectively, are displayed (Vem, 2.5 μM). (E) Western blot analysis for pERK and tERK of the protein lysate at the indicated concentrations of vemurafenib and trametinib (1 μM) for patient 4. The experiment was performed 3 times with similar results. (F) Quantification of the pERK/tERK ratios and pERK/14-3-3 ratios of all analyzed patients (n = 4) are displayed (Vem, 2.5 μM).
Figure 3. BRAF inhibition increases ERK phosphorylation and CLL proliferation in vivo, which can be reverted by SYK inhibition.
Highly purified (>97%) CD19+CD5+ cells (CLL) obtained from the patient were exposed to dabrafenib (6 μM) and R406 at increasing concentrations, as indicated, or DMSO as control. (A) A representative Western blot is shown. The experiment was performed twice with similar results. The pERK/tERK ratios (B) and the pSYK/tSYK ratios (C) are shown for the indicated conditions. (D) The patient’s CLL cells were exposed to dabrafenib, vemurafenib, R406, or DMSO as control at the indicated concentrations, and the resulting Western blot and the RAS-GTP/tRAS ratios are shown. One of 3 independent experiments with similar results is shown. (E) The amounts of CLL cells in the peripheral blood of Rag2–/– γC–/– mice on day 7 relative to treatment starting with vemurafenib (24 mg/kg/d) alone or vemurafenib and fostamatinib (60 mg/kg/d) are shown. On day 0, 2.5 × 107 patient-derived CD19+CD5+ cells were injected i.p. and i.v. (F) The survival of the Rag2–/– γC–/– mice treated as described in E is shown (P = 0.0001). Data from 2 experiments were pooled.
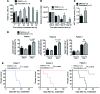
Figure 2. Impact of BRAF and MEK inhibitor treatment on signaling and survival of CLL cells.
Highly purified (>97%) CD19+CD5+ cells or CD14+ myeloid cells obtained from the patient were cultured in the presence of the BRAF inhibitors (vemurafenib, dabrafenib), the MEK inhibitor trametinib, BRAF inhibitor combined with MEK inhibitor, or DMSO only at the indicated concentrations. (A) OD as an indicator for viability and metabolic activity of CLL cells under different vemurafenib concentrations in an MTT assay. The experiment was performed twice using in dependent samples from the patient with similar results. Levels of pERK and tERK in the CLL cells (B) or CD14+ cells (C) derived from PBMC were measured by Western blot. We used vemurafenib, dabrafenib, and trametinib (0.07 μM) as indicated. One of 3 independent experiments with similar results is shown. (D) Western blot analysis for pERK and tERK of the protein lysate of highly purified (>97%) patient-derived CD19+CD5+ cells at the indicated concentrations of dabrafenib (Dab; 6 μM) and trametinib (MEK-i; 0.03, 0.07, 0.14 μM). Quantification of the protein amount of the described groups shown as a bar diagram. The experiment was performed 3 times with similar results. (E) The amount of CLL cells in the peripheral blood of Rag2–/– γC–/– mice relative to start of treatment with vehicle or vemurafenib (24 mg/kg/d). (F) The survival of the Rag2–/– γC–/– mice treated as described in panel E is shown (P = 0.0004). Data from 3 independent experiments were pooled.
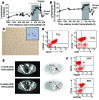
Figure 1. Clinical course of a melanoma patient with CLL progressing during treatment with vemurafenib.
Displayed are the white-cell count (A) and the lymphocyte count (B) at multiple time points prior to and after vemurafenib treatment (gray area). (C) A representative blood smear of the patient during vemurafenib treatment is shown. Original magnification, ×100; ×200 (inset). The dominant population has a mature lymphocyte phenotype. (D) Immunophenotyping of the white blood cells during vemurafenib treatment revealed a CD19+CD200+ population that could also be seen as CD19+CD5+ cells. (E) Combined CT and FDG PET scans obtained 1 month before the patient started taking vemurafenib and 6 weeks after vemurafenib was discontinued showed a partial response to treatment. The maximal standardized uptake value (SUVmax; semi-quantitative measure of tumor glucose metabolism) decreased from 9.5 to 4.6 g/ml (–52%) in the parailiacal LN (red arrow) and from 6.6 g/ml to 1.9 g/ml (–71%) in the right inguinal LN (not shown). Arrows indicate melanoma metastasis. (F) Lambda light-chain restriction in CD19+ B lymphocytes on day 732 relative to treatment initiation is shown.
Comment in
- Shifting ecologies of malignant and nonmalignant cells following BRAF inhibition.
Wu CJ. Wu CJ. J Clin Invest. 2014 Nov;124(11):4681-3. doi: 10.1172/JCI78783. Epub 2014 Oct 20. J Clin Invest. 2014. PMID: 25329690 Free PMC article.
Similar articles
- Molecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer.
Johnson GL, Stuhlmiller TJ, Angus SP, Zawistowski JS, Graves LM. Johnson GL, et al. Clin Cancer Res. 2014 May 15;20(10):2516-22. doi: 10.1158/1078-0432.CCR-13-1081. Epub 2014 Mar 24. Clin Cancer Res. 2014. PMID: 24664307 Free PMC article. - Efficacy of intermittent combined RAF and MEK inhibition in a patient with concurrent BRAF- and NRAS-mutant malignancies.
Abdel-Wahab O, Klimek VM, Gaskell AA, Viale A, Cheng D, Kim E, Rampal R, Bluth M, Harding JJ, Callahan MK, Merghoub T, Berger MF, Solit DB, Rosen N, Levine RL, Chapman PB. Abdel-Wahab O, et al. Cancer Discov. 2014 May;4(5):538-45. doi: 10.1158/2159-8290.CD-13-1038. Epub 2014 Mar 3. Cancer Discov. 2014. PMID: 24589925 Free PMC article. - Progression of RAS-mutant leukemia during RAF inhibitor treatment.
Callahan MK, Rampal R, Harding JJ, Klimek VM, Chung YR, Merghoub T, Wolchok JD, Solit DB, Rosen N, Abdel-Wahab O, Levine RL, Chapman PB. Callahan MK, et al. N Engl J Med. 2012 Dec 13;367(24):2316-21. doi: 10.1056/NEJMoa1208958. Epub 2012 Nov 7. N Engl J Med. 2012. PMID: 23134356 Free PMC article. - Vemurafenib.
Garbe C, Abusaif S, Eigentler TK. Garbe C, et al. Recent Results Cancer Res. 2014;201:215-25. doi: 10.1007/978-3-642-54490-3_13. Recent Results Cancer Res. 2014. PMID: 24756795 Review. - Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.
Ravnan MC, Matalka MS. Ravnan MC, et al. Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
Cited by
- Resolution of lung adenocarcinoma after discontinuation of ibrutinib.
Khashab T, Loghavi S, Konoplev SN, Samaniego F. Khashab T, et al. BMJ Case Rep. 2016 Jul 18;2016:bcr2016215342. doi: 10.1136/bcr-2016-215342. BMJ Case Rep. 2016. PMID: 27435843 Free PMC article. - KRAS, NRAS, and BRAF mutations are highly enriched in trisomy 12 chronic lymphocytic leukemia and are associated with shorter treatment-free survival.
Vendramini E, Bomben R, Pozzo F, Benedetti D, Bittolo T, Rossi FM, Dal Bo M, Rabe KG, Pozzato G, Zaja F, Chiarenza A, Di Raimondo F, Braggio E, Parikh SA, Kay NE, Shanafelt TD, Del Poeta G, Gattei V, Zucchetto A. Vendramini E, et al. Leukemia. 2019 Aug;33(8):2111-2115. doi: 10.1038/s41375-019-0444-6. Epub 2019 Mar 14. Leukemia. 2019. PMID: 30872781 Free PMC article. No abstract available. - Discrete cytosolic macromolecular BRAF complexes exhibit distinct activities and composition.
Diedrich B, Rigbolt KT, Röring M, Herr R, Kaeser-Pebernard S, Gretzmeier C, Murphy RF, Brummer T, Dengjel J. Diedrich B, et al. EMBO J. 2017 Mar 1;36(5):646-663. doi: 10.15252/embj.201694732. Epub 2017 Jan 16. EMBO J. 2017. PMID: 28093501 Free PMC article. - BRAFV600E induces ABCB1/P-glycoprotein expression and drug resistance in B-cells via AP-1 activation.
Tsai YT, Lozanski G, Lehman A, Sass EJ, Hertlein E, Salunke SB, Chen CS, Grever MR, Byrd JC, Lucas DM. Tsai YT, et al. Leuk Res. 2015 Sep 5:S0145-2126(15)30371-4 10.1016/j.leukres.2015.08.017. doi: 10.1016/j.leukres.2015.08.017. Online ahead of print. Leuk Res. 2015. PMID: 26350141 Free PMC article. - Hairy cell leukemia 2018: Update on diagnosis, risk-stratification, and treatment.
Troussard X, Cornet E. Troussard X, et al. Am J Hematol. 2017 Dec;92(12):1382-1390. doi: 10.1002/ajh.24936. Am J Hematol. 2017. PMID: 29110361 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous