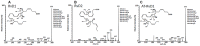Plasma metabolomics in human pulmonary tuberculosis disease: a pilot study - PubMed (original) (raw)
. 2014 Oct 15;9(10):e108854.
doi: 10.1371/journal.pone.0108854. eCollection 2014.
Dean P Jones 1, Nestan Tukvadze 2, Karan Uppal 3, Eka Sanikidze 2, Maia Kipiani 2, ViLinh T Tran 3, Gautam Hebbar 3, Douglas I Walker 4, Russell R Kempker 3, Shaheen S Kurani 3, Romain A Colas 5, Jesmond Dalli 5, Vin Tangpricha 6, Charles N Serhan 5, Henry M Blumberg 7, Thomas R Ziegler 1
Affiliations
- PMID: 25329995
- PMCID: PMC4198093
- DOI: 10.1371/journal.pone.0108854
Plasma metabolomics in human pulmonary tuberculosis disease: a pilot study
Jennifer K Frediani et al. PLoS One. 2014.
Abstract
We aimed to characterize metabolites during tuberculosis (TB) disease and identify new pathophysiologic pathways involved in infection as well as biomarkers of TB onset, progression and resolution. Such data may inform development of new anti-tuberculosis drugs. Plasma samples from adults with newly diagnosed pulmonary TB disease and their matched, asymptomatic, sputum culture-negative household contacts were analyzed using liquid chromatography high-resolution mass spectrometry (LC-MS) to identify metabolites. Statistical and bioinformatics methods were used to select accurate mass/charge (m/z) ions that were significantly different between the two groups at a false discovery rate (FDR) of q<0.05. Two-way hierarchical cluster analysis (HCA) was used to identify clusters of ions contributing to separation of cases and controls, and metabolomics databases were used to match these ions to known metabolites. Identity of specific D-series resolvins, glutamate and Mycobacterium tuberculosis (Mtb)-derived trehalose-6-mycolate was confirmed using LC-MS/MS analysis. Over 23,000 metabolites were detected in untargeted metabolomic analysis and 61 metabolites were significantly different between the two groups. HCA revealed 8 metabolite clusters containing metabolites largely upregulated in patients with TB disease, including anti-TB drugs, glutamate, choline derivatives, Mycobacterium tuberculosis-derived cell wall glycolipids (trehalose-6-mycolate and phosphatidylinositol) and pro-resolving lipid mediators of inflammation, known to stimulate resolution, efferocytosis and microbial killing. The resolvins were confirmed to be RvD1, aspirin-triggered RvD1, and RvD2. This study shows that high-resolution metabolomic analysis can differentiate patients with active TB disease from their asymptomatic household contacts. Specific metabolites upregulated in the plasma of patients with active TB disease, including Mtb-derived glycolipids and resolvins, have potential as biomarkers and may reveal pathways involved in TB disease pathogenesis and resolution.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
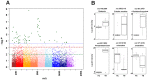
Figure 1. Plasma metabolome-wide association study (MWAS) of pulmonary tuberculosis (TB) disease in adults.
(A) The Manhattan plot depicts the -log P analysis of 23,241 metabolites comparing 17 adults with newly diagnosed pulmonary TB disease and 17 asymptomatic adult household contacts who were sputum smear and culture negative for Mtb. The x-axis represents the m/z of the metabolites, ordered in increasing value from left (85) to right (2000). A total of 61 metabolites significantly differed [false discovery rate (FDR) q = 0.05] between the two groups (metabolites depicted above the horizontal dashed blue line). Metabolites above the horizontal dashed red line (n = 122) distinguished the TB disease and household contact cohorts at FDR q = 0.10, while metabolites above the dashed green line (n = 711) distinguished the two groups at FDR q = 0.20. (B) Box-and-whisker plots of log2 intensities comparing individuals with TB disease and household contacts for selected metabolites, with m/z and putative metabolite identification from Metlin and KEGG (left to right upper panel: glutamate, D-series resolvin, and trehalose-6-mycolate, respectively; left to right lower panel: phosphatidylinositol, and two unidentified metabolites that did not match to known metabolites in the databases, respectively).
Figure 2. Significant metabolites that distinguish TB patients from household contacts.
(A) Two-way hierarchical cluster analysis (HCA) using C18 chromatography shows 8 clusters of metabolites from human plasma and illustrates the patterns distinguishing those with active TB from household contacts without evidence of TB disease. The 17 subjects with TB disease (TB; shown in green) and the 17 household contacts (HC; shown in red) are shown along the x-axis. (B) Pie chart depicts chemical classes of the 61 significant metabolites from panel 2A according to high-resolution matches to metabolite databases .
Figure 3. MS/MS fragmentation spectra show positive identification of resolvin D1 (RvD1), resolvin D2 (RvD2), and aspirin-triggered resolvin D1 (AT-RvD1) in plasma from subjects with TB disease.
Metabololipidomics analytical methods that incorporated high-resolution liquid chromatography coupled with tandem mass spectroscopy (LC-MS/MS, ABI 5500, see methods) were used to verify these DHA-derived specialized pro-resolving lipid mediators , .
Similar articles
- High-resolution plasma metabolomics analysis to detect Mycobacterium tuberculosis-associated metabolites that distinguish active pulmonary tuberculosis in humans.
Collins JM, Walker DI, Jones DP, Tukvadze N, Liu KH, Tran VT, Uppal K, Frediani JK, Easley KA, Shenvi N, Khadka M, Ortlund EA, Kempker RR, Blumberg HM, Ziegler TR. Collins JM, et al. PLoS One. 2018 Oct 11;13(10):e0205398. doi: 10.1371/journal.pone.0205398. eCollection 2018. PLoS One. 2018. PMID: 30308073 Free PMC article. - Urinary biomarkers of mycobacterial load and treatment response in pulmonary tuberculosis.
Xia Q, Lee MH, Walsh KF, McAulay K, Bean JM, Fitzgerald DW, Dupnik KM, Johnson WD, Pape JW, Rhee KY, Isa F. Xia Q, et al. JCI Insight. 2020 Sep 17;5(18):e136301. doi: 10.1172/jci.insight.136301. JCI Insight. 2020. PMID: 32809976 Free PMC article. - A metabolic biosignature of early response to anti-tuberculosis treatment.
Mahapatra S, Hess AM, Johnson JL, Eisenach KD, DeGroote MA, Gitta P, Joloba ML, Kaplan G, Walzl G, Boom WH, Belisle JT. Mahapatra S, et al. BMC Infect Dis. 2014 Jan 31;14:53. doi: 10.1186/1471-2334-14-53. BMC Infect Dis. 2014. PMID: 24484441 Free PMC article. - Delamanid: From discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB).
Liu Y, Matsumoto M, Ishida H, Ohguro K, Yoshitake M, Gupta R, Geiter L, Hafkin J. Liu Y, et al. Tuberculosis (Edinb). 2018 Jul;111:20-30. doi: 10.1016/j.tube.2018.04.008. Epub 2018 May 3. Tuberculosis (Edinb). 2018. PMID: 30029909 Review. - Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.
Wolahan SM, Hirt D, Glenn TC. Wolahan SM, et al. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. PMID: 26269925 Free Books & Documents. Review.
Cited by
- HIV and the tuberculosis "set point": how HIV impairs alveolar macrophage responses to tuberculosis and sets the stage for progressive disease.
Auld SC, Staitieh BS. Auld SC, et al. Retrovirology. 2020 Sep 23;17(1):32. doi: 10.1186/s12977-020-00540-2. Retrovirology. 2020. PMID: 32967690 Free PMC article. Review. - Discovery of serum biomarkers for diagnosis of tuberculosis by NMR metabolomics including cross-validation with a second cohort.
Conde R, Laires R, Gonçalves LG, Rizvi A, Barroso C, Villar M, Macedo R, Simões MJ, Gaddam S, Lamosa P, Puchades-Carrasco L, Pineda-Lucena A, Patel AB, Mande SC, Banerjee S, Matzapetakis M, Coelho AV. Conde R, et al. Biomed J. 2022 Aug;45(4):654-664. doi: 10.1016/j.bj.2021.07.006. Epub 2021 Jul 24. Biomed J. 2022. PMID: 34314900 Free PMC article. - Untargeted metabolomics of pulmonary tuberculosis patient serum reveals potential prognostic markers of both latent infection and outcome.
Wang X, Wu Z, Zeng J, Zhao Y, Zhang C, Yu M, Wang W, Chen X, Chen L, Wang J, Xu L, Zhou J, Tan Q, Wei W, Li Y. Wang X, et al. Front Public Health. 2022 Nov 15;10:962510. doi: 10.3389/fpubh.2022.962510. eCollection 2022. Front Public Health. 2022. PMID: 36457328 Free PMC article. - Towards Mass Spectrometry-Based Chemical Exposome: Current Approaches, Challenges, and Future Directions.
Xue J, Lai Y, Liu CW, Ru H. Xue J, et al. Toxics. 2019 Aug 18;7(3):41. doi: 10.3390/toxics7030041. Toxics. 2019. PMID: 31426576 Free PMC article. Review. - Mass Spectrometric Identification of Urinary Biomarkers of Pulmonary Tuberculosis.
Isa F, Collins S, Lee MH, Decome D, Dorvil N, Joseph P, Smith L, Salerno S, Wells MT, Fischer S, Bean JM, Pape JW, Johnson WD, Fitzgerald DW, Rhee KY. Isa F, et al. EBioMedicine. 2018 May;31:157-165. doi: 10.1016/j.ebiom.2018.04.014. Epub 2018 Apr 22. EBioMedicine. 2018. PMID: 29752217 Free PMC article. Clinical Trial.
References
- World Health Organization (2013) WHO Global Report.
- Zumla A, George A, Sharma V, Herbert N (2013) Baroness Masham of Ilton (2013) WHO's 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet 382: 1765–1767. - PubMed
- Zumla A, Raviglione M, Hafner R, von Reyn CF (2013) Tuberculosis. New England Journal of Medicine 368: 745–755. - PubMed
- Maertzdorf J, Weiner JR, Kaufmann SH (2012) Enabling biomarkers for tuberculosis control. International Journal of Tuberculosis and Lung Disease 16: 1140–1148. - PubMed
- Wallis RS, Kim PC, Cole S, Hanna D, Andrade BB, et al. (2013) Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infectious Disease 13: 362–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20HL113451/HL/NHLBI NIH HHS/United States
- K23 AR054334/AR/NIAMS NIH HHS/United States
- T32 ES012870/ES/NIEHS NIH HHS/United States
- P01 ES016731/ES/NIEHS NIH HHS/United States
- D43 TW007124/TW/FIC NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- K23 AI103044/AI/NIAID NIH HHS/United States
- R01 ES016731/ES/NIEHS NIH HHS/United States
- P30 ES019776/ES/NIEHS NIH HHS/United States
- P20 HL113451/HL/NHLBI NIH HHS/United States
- R01 AG038746/AG/NIA NIH HHS/United States
- P30ES019776/ES/NIEHS NIH HHS/United States
- K24 DK096574/DK/NIDDK NIH HHS/United States
- P01 GM095467/GM/NIGMS NIH HHS/United States
- HHSN272201200031C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources