Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin - PubMed (original) (raw)
Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin
Suk Yu Yau et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Adiponectin (ADN) is an adipocyte-secreted protein with insulin-sensitizing, antidiabetic, antiinflammatory, and antiatherogenic properties. Evidence is also accumulating that ADN has neuroprotective activities, yet the underlying mechanism remains elusive. Here we show that ADN could pass through the blood-brain barrier, and elevating its levels in the brain increased cell proliferation and decreased depression-like behaviors. ADN deficiency did not reduce the basal hippocampal neurogenesis or neuronal differentiation but diminished the effectiveness of exercise in increasing hippocampal neurogenesis. Furthermore, exercise-induced reduction in depression-like behaviors was abrogated in ADN-deficient mice, and this impairment in ADN-deficient mice was accompanied by defective running-induced phosphorylation of AMP-activated protein kinase (AMPK) in the hippocampal tissue. In vitro analyses indicated that ADN itself could increase cell proliferation of both hippocampal progenitor cells and Neuro2a neuroblastoma cells. The neurogenic effects of ADN were mediated by the ADN receptor 1 (ADNR1), because siRNA targeting ADNR1, but not ADNR2, inhibited the capacity of ADN to enhance cell proliferation. These data suggest that adiponectin may play a significant role in mediating the effects of exercise on hippocampal neurogenesis and depression, possibly by activation of the ADNR1/AMPK signaling pathways, and also raise the possibility that adiponectin and its agonists may represent a promising therapeutic treatment for depression.
Keywords: adiponectin; adiponectin receptor; depression-like behavior; hippocampal neurogenesis; physical exercise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
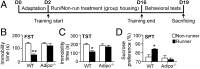
Elevation of adiponectin levels in the brain reduced depressive phenotype in mice. (A) Experimental timeline for administration of recombinant adenovirus and behavioral tests. C57BL/6J mice with i.c.v. injection of recombinant Ad-Adn or control Ad-Luc received the nonrunning treatment for 2 wk, followed by behavioral tests. (B) The tail TST and (C) the FST assessed the duration of immobility that parallels depressive severity. (D) The SPT examined the loss of preference to sucrose (anhedonia), a core symptom in depression. (E) Cell proliferation in the hippocampal dentate gyrus was determined by measuring the density of Ki67+ cells. *P < 0.05, **P < 0.005; n = 8 mice per group. (F and G) The immobility time of TST and FST was negatively correlated with the density of hippocampal Ki67+ cells. (H) Trimeric adiponectin (ADN, 20 μg) administered through the tail vein became detectable in CSF in _adipo_−/− mice at 3 h after injection. VEH, PBS as vehicle. **P < 0.005; n = 4 mice per group.
Fig. 2.
Depressive phenotype of WT and adipo −/− mice after the 2-wk running. (A) Experimental timeline for behavioral tests and sample collections. WT or adipo −/− mice received the 2-wk nonrunning or running treatment, followed by behavioral tests, including FST (B), TST (C), and SPT (D). Depression-like behaviors were significantly reduced by running in WT, but not adipo −/− mice. *P < 0.05 and **P < 0.005 vs. WT nonrunners; n = 8–10 mice per group.
Fig. 3.
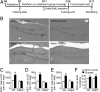
Cellular changes in the hippocampus of WT and adipo −/− mice after exercise. (A) Experimental timeline for the running or nonrunning treatment, FST, and immunohistochemical analyses. WT and adipo −/− mice receiving the nonrunning or running treatment were daily injected with BrdU to label newborn cells during the last 5 consecutive days of the 2-wk training period. (B) Representative images of newborn (BrdU+) cells in the hippocampal dentate gyrus. (Scale bars, 100 µm.) (C and D) Running-enhanced hippocampal cell proliferation, reflected by the density of BrdU+ or Ki67+ population, was observed in WT but not adipo −/− mice. (E) Adiponectin knockout also diminished the effect of running on increasing the number of immature neurons (DCX+), without affecting the baseline. *P < 0.005 vs. WT nonrunners. (F) Neuronal differentiation estimated by the colabeling ratio of BrdU and DCX was comparable between WT and adipo −/− mice receiving the same treatments. *P < 0.05 vs. WT nonrunners, **P < 0.01 vs. adipo −/− nonrunners; n = 5–6 mice per group.
Fig. 4.
Effects of running on neurotrophic factors. The brain samples collected from adipo −/− mice or WT littermates receiving the 2-wk running or nonrunning treatment as described in Fig. 2_A_ were homogenized and subjected to immunoassays. (A and B) Running significantly increased the hippocampal but not circulating adiponectin levels in WT mice. *P < 0.05 vs. WT nonrunners. Note that adiponectin was undetectable in either the serum or hippocampal lysate of Adipo−/− mice. (C and D) The levels of BDNF in the whole hippocampus were unaffected by either exercise or adiponectin knockout. Running raised the BDNF levels specifically in the dentate gyrus of WT and adipo −/− mice essentially to the same extent. (E and F) adipo −/− mice showed lower protein levels of IGF-1 in the whole hippocampus compared with WT animals (*main effect of genotype: P < 0.001), whereas IGF-1 levels in the dentate region remained comparable in all four groups. n = 4–6 mice per group.
Fig. 5.
Effects of running and adiponectin on several signaling pathways in the hippocampus. The homogenates of hippocampal tissues collected from adipo −/− mice or WT littermates receiving the 2-wk running or nonrunning treatment as described in Fig. 2_A_ were subjected to Western blot analysis. (A) Representative immunoblotting images for phospho-AMPK (T172), total AMPK, phospho-Akt (S473), total Akt, phospho-Erk1/2 (T202/Y204), total Erk1/2, phospho-p38MAPK (T180/Y182), total p38MAPK, and the loading control β-actin. (B–E) Semiquantitative analysis for phosphor-AMPK (T172), phospho-Akt (S473), phospho-Erk1/2 (T202/Y204), and phospho-p38MAPK (T180/Y182). The data were expressed as fold changes over WT nonrunners. *P < 0.005 vs. WT nonrunners. n = 9 mice per group.
Fig. 6.
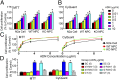
Adiponectin-induced enhancement of cell proliferation was mediated by ADNR1. The N2a cell line as well as the primary NPCs isolated from WT (WT NPC) and adipo −/− mice (KO NPC) were incubated with different concentrations of trimeric adiponectin and measured for proliferation using MTT (A and C, Left) and CyQuant assays (B and C, Right). *P < 0.05 vs. control cells without adiponectin treatment. (C) The concentration-dependent curves replotted using the data from A and B show the comparable responses to adiponectin in these three cell preparations. (D) Down-regulating ADNR1 but not ADNR2 in N2a cells with siRNA abolished adiponectin-enhanced proliferation. *P < 0.05 vs. Scramble siRNA-transfected cells (SC) without adiponectin treatment. n = 4–5 independent experiments for each assay. Numbers enclosed in the bracelets indicate the concentrations of the trimeric adiponectin applied. CT, combined transfection of si-Adnr1 and si-Adnr2.
Comment in
- Neuroendocrinology: Role for adiponectin in effects of exercise in depression.
Greenhill C. Greenhill C. Nat Rev Endocrinol. 2015 Jan;11(1):4. doi: 10.1038/nrendo.2014.201. Epub 2014 Nov 11. Nat Rev Endocrinol. 2015. PMID: 25385036 No abstract available.
Similar articles
- Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress.
Liu L, Tang J, Liang X, Li Y, Zhu P, Zhou M, Qin L, Deng Y, Li J, Wang Y, Jiang L, Huang D, Zhou Y, Wang S, Xiao Q, Luo Y, Tang Y. Liu L, et al. Mol Psychiatry. 2024 Jul;29(7):2031-2042. doi: 10.1038/s41380-024-02464-1. Epub 2024 Feb 15. Mol Psychiatry. 2024. PMID: 38361125 - Fat cell-secreted adiponectin mediates physical exercise-induced hippocampal neurogenesis: an alternative anti-depressive treatment?
Yau SY, Li A, Xu A, So KF. Yau SY, et al. Neural Regen Res. 2015 Jan;10(1):7-9. doi: 10.4103/1673-5374.150637. Neural Regen Res. 2015. PMID: 25788905 Free PMC article. Review. - Mechanisms underpinning AMP-activated protein kinase-related effects on behavior and hippocampal neurogenesis in an animal model of depression.
Odaira T, Nakagawasai O, Takahashi K, Nemoto W, Sakuma W, Lin JR, Tan-No K. Odaira T, et al. Neuropharmacology. 2019 May 15;150:121-133. doi: 10.1016/j.neuropharm.2019.03.026. Epub 2019 Mar 23. Neuropharmacology. 2019. PMID: 30914305 - Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice.
Wang P, Liang Y, Chen K, Yau SY, Sun X, Cheng KK, Xu A, So KF, Li A. Wang P, et al. Front Cell Neurosci. 2020 Jul 3;14:189. doi: 10.3389/fncel.2020.00189. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32774242 Free PMC article. - Adult Neurogenic and Antidepressant Effects of Adiponectin: A Potential Replacement for Exercise?
Li A, Yau SY, Machado S, Yuan TF, So KF. Li A, et al. CNS Neurol Disord Drug Targets. 2015;14(9):1129-44. doi: 10.2174/1871527315666151111125533. CNS Neurol Disord Drug Targets. 2015. PMID: 26556072 Review.
Cited by
- A brief review of exercise, bipolar disorder, and mechanistic pathways.
Thomson D, Turner A, Lauder S, Gigler ME, Berk L, Singh AB, Pasco JA, Berk M, Sylvia L. Thomson D, et al. Front Psychol. 2015 Mar 4;6:147. doi: 10.3389/fpsyg.2015.00147. eCollection 2015. Front Psychol. 2015. PMID: 25788889 Free PMC article. - Is Adult Hippocampal Neurogenesis Really Relevant for the Treatment of Psychiatric Disorders?
Carli M, Aringhieri S, Kolachalam S, Longoni B, Grenno G, Rossi M, Gemignani A, Fornai F, Maggio R, Scarselli M. Carli M, et al. Curr Neuropharmacol. 2021;19(10):1640-1660. doi: 10.2174/1570159X18666200818194948. Curr Neuropharmacol. 2021. PMID: 32811415 Free PMC article. Review. - Increasing Adiponectin Signaling by Sub-Chronic AdipoRon Treatment Elicits Antidepressant- and Anxiolytic-Like Effects Independent of Changes in Hippocampal Plasticity.
Formolo DA, Lee TH, Yu J, Lin K, Chen G, Kranz GS, Yau SY. Formolo DA, et al. Biomedicines. 2023 Jan 18;11(2):249. doi: 10.3390/biomedicines11020249. Biomedicines. 2023. PMID: 36830788 Free PMC article. - Associations of cord blood leptin and adiponectin with children's cognitive abilities.
Li N, Arbuckle TE, Muckle G, Lanphear BP, Boivin M, Chen A, Dodds L, Fraser WD, Ouellet E, Séguin JR, Velez MP, Yolton K, Braun JM. Li N, et al. Psychoneuroendocrinology. 2019 Jan;99:257-264. doi: 10.1016/j.psyneuen.2018.10.021. Epub 2018 Oct 25. Psychoneuroendocrinology. 2019. PMID: 30390444 Free PMC article. - Adipose transplantation improves olfactory function and neurogenesis via PKCα-involved lipid metabolism in Seipin Knockout mice.
Yang J, Yang N, Zhao H, Qiao Y, Li Y, Wang C, Lim KL, Zhang C, Yang W, Lu L. Yang J, et al. Stem Cell Res Ther. 2023 Sep 7;14(1):239. doi: 10.1186/s13287-023-03463-9. Stem Cell Res Ther. 2023. PMID: 37674230 Free PMC article.
References
- Rimer J, et al. Exercise for depression. Cochrane Database Syst Rev. 2012;7:CD004366. - PubMed
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous