Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression - PubMed (original) (raw)
Don L Gibbons # 1 3, Sangeeta Goswami 1, Maria Angelica Cortez 1, Young-Ho Ahn 1 4, Lauren A Byers 1, Xuejun Zhang 2, Xiaohui Yi 2, David Dwyer 2, Wei Lin 1, Lixia Diao 5, Jing Wang 5, Jonathon Roybal 1, Mayuri Patel 1, Christin Ungewiss 1, David Peng 1, Scott Antonia 6, Melanie Mediavilla-Varela 6, Gordon Robertson 7, Milind Suraokar 1 8, James W Welsh 9, Baruch Erez 1, Ignacio I Wistuba 1 8, Lieping Chen 10, Di Peng 11, Shanshan Wang 11, Stephen E Ullrich 2, John V Heymach 1, Jonathan M Kurie 1, F Xiao-Feng Qin 1 2 11
Affiliations
- PMID: 25348003
- PMCID: PMC4212319
- DOI: 10.1038/ncomms6241
Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression
Limo Chen et al. Nat Commun. 2014.
Abstract
Immunosuppression of tumour-infiltrating lymphocytes (TIL) is a common feature of advanced cancer, but its biological basis has remained obscure. We demonstrate here a molecular link between epithelial-to-mesenchymal transition (EMT) and CD8(+) TIL immunosuppression, two key drivers of cancer progression. We show that microRNA-200 (miR-200), a cell-autonomous suppressor of EMT and metastasis, targets PD-L1. Moreover, ZEB1, an EMT activator and transcriptional repressor of miR-200, relieves miR-200 repression of PD-L1 on tumour cells, leading to CD8(+) T-cell immunosuppression and metastasis. These findings are supported by robust correlations between the EMT score, miR-200 levels and PD-L1 expression in multiple human lung cancer datasets. In addition to revealing a link between EMT and T-cell dysfunction, these findings also show that ZEB1 promotes metastasis through a heretofore unappreciated cell non-autonomous mechanism, and suggest that subgroups of patients in whom malignant progression is driven by EMT activators may respond to treatment with PD-L1 antagonists.
Figures
Figure 1. EMT correlates with miR-200 and PD-L1 expression in clinical lung cancer datasets
(a) Application of the EMT score (red (right) represents mesenchymal and blue (left) is epithelial) to the lung adenocarcinoma samples from the TCGA dataset (n=230) stratifies the samples by miR-200 family levels (top), EMT marker expression by RNA sequencing (middle), and protein markers (bottom). (b) Dot plot of the concordant EMT score and PD-L1 mRNA expression levels from the samples in the LUAD TCGA. The Spearman correlation is 0.527, p < 0.0001. (c) Dot plot of the concordant PD-L1 (CD274) and miR-200b expression levels from the TCGA samples. The Spearman correlation is −0.454, p < 0.0001. (d) Application of the EMT score to the samples from the PROSPECT dataset. Samples in the top 1/3 were designated mesenchymal (M) and in the bottom 1/3 designated as epithelial (E). Each group has n=92 and is represented in the box plot format. The PD-L1 mRNA expression level is graphed for the samples in each group. _t_-test, p < 0.0001.
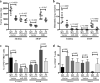
Figure 2. PD-L1 levels on human and murine NSCLC cell lines are regulated by the miR-200/ZEB1 axis
(a) Representative FACS histogram of PD-L1 expression on murine (344SQ, 531LN2, and 393P) and human (H157, H1155, and HCC827) lung cancer cells by overexpression of miR-200 and Zeb1. The analysis was independently repeated three times. (b) The representative FACS histogram of PD-L1 expression on murine lung cancer cells co-cultured with 129/Sv murine splenocytes with or without IFN-γ neutralizing antibody. Long dash is isotype control staining; blue line is anti-PD-L1 staining without splenocytes; green line is anti-PD-L1 staining with splenocytes blocked by the neutralizing antibody to IFN-γ (20 μg ml−1, 48 hrs); red line is anti-PD-L1 staining with splenocytes (48 hrs). The assay was independently performed three times. (c) The representative FACS histogram of PD-L1 (MFI, mean fluorescence intensity) expression in primary subcutaneous tumors grown in syngeneic 129/Sv mice (n = 3) injected with the indicated cell lines shown in the upper panel. The representative PD-L1 IHC staining of each tumor type shown in the bottom panel. Samples were obtained 2 weeks post-cell injection. Scale bar, 100 μm. (d) 3’-UTR luciferase reporter assay for wild-type PD-L1, single (mutant A or B) and double mutants (A and B) versus a Zeb1 3’-UTR control in murine 344SQ cells. The reporters were transiently co-transfected with synthetic miR-200 precursors (200a, 200b, or 200c) or control oligomers (con) into 344SQ cells. Values (p < 0.05) were normalized based on renilla luciferase and expressed as the mean values (±S.D.) of triplicate wells relative to that of controls co-transfected with empty reporter and empty expression vector or scrambled precursors, which were set at 1.0. The target sequences are shown in the top panel, with the introduced mutations highlighted in red.
Figure 3. CD8+TILs determine the metastatic potential in lung adenocarcinoma models
(a) CD8+ T cells measured by flow cytometric analysis in single-cell suspensions prepared from tumor-bearing lungs of 8- to 12- month-old K-rasLA1/+ (K-ras) and K-rasLA1/+p53R172HΔG/+ (KP) spontaneous mouse model (n = 5). The analysis was independently repeated at least three times. _t_-test, p = 0.0006. (b) The results of a FACS analysis of CD8+TILs in 344SQ and 393P primary tumors isolated 2 weeks after subcutaneous tumor cell injection into 129/Sv mice (n = 10). The results contain the data from two independent experiments. _t_-test, p < 0.0001. (c) Representative flow cytometric plot of CD8+TIL numbers from 393P tumor-bearing 129/Sv mice (n = 5) treated with anti-CD8 or IgG control antibodies (200 μg, intraperitoneally; twice weekly for 2 weeks beginning on day 1 after a subcutaneous cancer cell injection). (d) Lung metastases in WT or 129/Sv_Rag2_−/− mice (n = 5) injected subcutaneously with 344SQ or 393P cells and necropsied 5 weeks later. The analysis was independently repeated twice. _t_-test, p < 0.0001. (e) CD8+ T cells isolated from 129/Sv mice were adoptively transferred to syngeneic 129/Sv_Rag2_−/− mice (n = 5). Representative FACS histograms depict the levels of CD8+ T cells in 344SQ tumors and lungs of the reconstituted mice with reconstituted CD8+ T cells versus those in the controls.
Figure 4. The miR-200/ZEB1 axis controls tumor metastasis through regulating CD8+TILs
(a, b) FACS analysis of (a) CD8+TIL frequency; (b) PD1 and TIM3 marker expression on CD8+ T cells from 393P_vector and 393P_ZEB1 (n = 5), as well as 344SQ_vector and 344SQ_miR-200 (n = 10) primary tumors. Analysis was done 2 weeks post-cancer cell injection. (c, d) (c) Intratumoral Ki67+CD8+ T cells; (d) granzyme B (GzB)+CD8+ T cells in 344SQ_vector or 344SQ_miR-200 primary tumors 6 weeks post-subcutaneous injection of cancer cells into 129/Sv mice. Representative Ki67 or GzB staining in an individual tumor sample is shown on the left, and mean Ki67+ or GzB+ populations of gated CD8+ T cells in total T cells are shown on the right (n = 5). (e) CD8+ T cell depletion results in tumor growth and metastasis in mice (n = 5) that received subcutaneous tumor cell injections. No treatment (344SQ_vector (Vector)), IgG (344SQ_miR-200 + IgG control), or Ab (344SQ_miR-200 + anti-CD8 Ab). The analysis was done 6 weeks post-injection. (f) Relative abundance of CD8+ T cells in the tumor (left) or lung (right) from 129/Sv mice (n =5) with syngeneic control 344SQ tumors (Vector), 344SQ_miR-200 tumors with control IgG treatment (IgG) or anti-CD8 antibody treatment (Ab). (g) Lung metastases of 344SQ_vector (Vector) and 344SQ_miR-200 (miR-200) tumors in wild-type (WT) or 129/Sv_Rag2−/−_ (Rag2−/−) mice (n = 5). The analysis was done 6 weeks post-tumor cell subcutaneous injection. All the analyses were independently repeated twice. Data are shown as mean ± s.e.m. _t_-test was used to analyze, with P values shown in the graphs.
Figure 5. Genetic targeting of PD-L1 expression on cancer cells reverses the CD8+TIL dysfunction and suppresses metastasis
(a) Cell surface expression of PD-L1 on 344SQ PD-L1 knockdown (344SQ-shPD-L1) vs 344SQ scramble control (344SQ-scr) cells by FACS (red line, isotype control staining; blue line, anti-PD-L1 staining). The measurement was independently repeated at least three times. (b) Primary tumor mass (top left) and lung metastases (top right) in 129/Sv mice (n = 10) injected subcutaneously with 344SQ-shPD-L1 or 344SQ-scr cancer cells. Micrometastases (bottom) observed in hematoxylin and eosin-stained lung tissue sections are indicated by yellow arrows. Scale bar, 2mm. Samples were obtained 6 weeks post-injection. The data from two independent experiments were pooled. Data are shown as mean ± s.e.m. _t_-test was used to analyze. P values are shown in the graphs. (c, d) FACS analysis of (c) surface PD1, LAG3, and TIM3 marker expression levels on CD8+ T cells; (d) CD8+TIL frequency for primary tumors in 129/Sv mice (n = 5) injected subcutaneously with 344SQ-shPD-L1 (shPD-L1) or 344SQ-scr control (Control) cancer cells and necropsied 2 weeks later. The analyses were independently repeated three times. Data are shown as mean ± s.e.m. _t_-test was used to analyze. P values are shown in the graphs.
Figure 6. Pharmacologic targeting of PD-L1 with antibody treatment prevents tumor growth and metastasis
(a and b) The tumor burden (a) and lung metastases (b) 6 weeks post-cancer cell injection into 129/Sv mice (n = 5) with IgG control (IgG) or anti-PD-L1 antibody (Ab) treatment. Anti-PD-L1 antibody or IgG control was injected (100 μg, intraperitoneally) twice a week for 6 weeks after the subcutaneous tumor cell injection. Following euthanasia, mice were necropsied to harvest primary tumors and lungs, which were weighed, and to quantify distant metastases. (c and d) The CD8+ T cell function and the abundance of CD8+ T cells in tumors (n = 5) after anti-PD-L1 antibody treatment. All the analyses were independently repeated twice. Data are shown as mean ± s.e.m. _t_-test was used to analyze. P values are shown in the graphs.
Figure 7. PD-L1 expression on tumor cells is critical to the repression of anti-tumor immunity
(a) The knockdown (KD) efficiency of PD-L1 in LLC-JSP murine lung cancer cells measured by FACS. Representative histograms (left), and statistical analysis (right). The measurement was independently repeated at least three times. (b) Representative FACS histogram of PD-L1 expression on myeloid cells (CD11b+) in PD-L1 KO or WT mice (n = 3). (c) Tumor growth in PD-L1 KO or WT mice (n = 6) of subcutaneously injected LLC-JSP cells (10, 000 cells with 100 μl of PBS per mouse) with differing PD-L1 knockdown (Vector, PD-L1 KD vector control; PD-L1int, PD-L1 intermediate KD; PD-L1hi, PD-L1 high level KD). The data is shown from two independent experiments. Data are shown as mean ± s.e.m. (d) FACS analysis of CD8+TIL frequency and T cell exhaustion marker expression levels on CD8+ T cells in subcutaneous primary tumors of LLC-JSP vector control (Vector ctrl) and LLC-JSP intermediate PD-L1 knockdown (Intermediate KD) in PD-L1 WT (WT) and PD-L1 KO (KO) mice (n = 5, from two independent experiments). Analysis was done 3 weeks post-tumor cell injection (20, 000 cells with 100 μl of PBS per mouse). _t_-test was used to analyze the data. p < 0.0001. (**e**) PD-L1 expression levels on LLC-JSP-shPD-L1 cells (the high knockdown efficiency cells) after reconstitution of PD-L1. The measurement was independently repeated at least three times. Control, LLC-JSP-shPD-L1 + vector control; PD-L1medium, LLC-JSP-shPD-L1 + intermediate overexpression of PD-L1; PD-L1high, LLC-JSP-shPD-L1 + high overexpression of PD-L1. (**f**) Primary tumor masses and lung metastases in PD-L1 KO or WT mice (n = 5) injected subcutaneously with the different reconstituted cell lines. Analysis was done 4 weeks post-subcutaneous cancer cell injection (10, 000 cells with 100 μl of PBS per mouse). The analyses were independently repeated twice. Data are shown as mean ± s.e.m. _t_-test was used to analyze the data. ns, p > 0.05. (g) The working model of the mutual regulation of EMT and immune suppression by miR-200/ZEB1 axis and their complementary role in metastasis.
Similar articles
- UBE2C, Directly Targeted by miR-548e-5p, Increases the Cellular Growth and Invasive Abilities of Cancer Cells Interacting with the EMT Marker Protein Zinc Finger E-box Binding Homeobox 1/2 in NSCLC.
Jin D, Guo J, Wu Y, Du J, Wang X, An J, Hu B, Kong L, Di W, Wang W. Jin D, et al. Theranostics. 2019 Mar 17;9(7):2036-2055. doi: 10.7150/thno.32738. eCollection 2019. Theranostics. 2019. PMID: 31037155 Free PMC article. Retracted. - PD-L1 knockdown suppresses vasculogenic mimicry of non-small cell lung cancer by modulating ZEB1-triggered EMT.
Li W, Wu J, Jia Q, Shi Y, Li F, Zhang L, Shi F, Wang X, Wu S. Li W, et al. BMC Cancer. 2024 May 23;24(1):633. doi: 10.1186/s12885-024-12390-8. BMC Cancer. 2024. PMID: 38783271 Free PMC article. - The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs.
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. Wellner U, et al. Nat Cell Biol. 2009 Dec;11(12):1487-95. doi: 10.1038/ncb1998. Epub 2009 Nov 22. Nat Cell Biol. 2009. PMID: 19935649 - Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma.
Tsutsumi S, Saeki H, Nakashima Y, Ito S, Oki E, Morita M, Oda Y, Okano S, Maehara Y. Tsutsumi S, et al. Cancer Sci. 2017 Jun;108(6):1119-1127. doi: 10.1111/cas.13237. Epub 2017 May 25. Cancer Sci. 2017. PMID: 28294486 Free PMC article. - The roles of PD-L1 in the various stages of tumor metastasis.
He Y, Zhu M, Lai X, Zhang H, Jiang W. He Y, et al. Cancer Metastasis Rev. 2024 Dec;43(4):1475-1488. doi: 10.1007/s10555-024-10189-4. Epub 2024 May 11. Cancer Metastasis Rev. 2024. PMID: 38733457 Review.
Cited by
- Signaling pathways and therapeutic interventions in gastric cancer.
Lei ZN, Teng QX, Tian Q, Chen W, Xie Y, Wu K, Zeng Q, Zeng L, Pan Y, Chen ZS, He Y. Lei ZN, et al. Signal Transduct Target Ther. 2022 Oct 8;7(1):358. doi: 10.1038/s41392-022-01190-w. Signal Transduct Target Ther. 2022. PMID: 36209270 Free PMC article. Review. - MicroRNAs Modulate Drug Resistance-Related Mechanisms in Hepatocellular Carcinoma.
Liang Y, Liang Q, Qiao L, Xiao F. Liang Y, et al. Front Oncol. 2020 Jun 30;10:920. doi: 10.3389/fonc.2020.00920. eCollection 2020. Front Oncol. 2020. PMID: 32695666 Free PMC article. Review. - PIAS1-FAK Interaction Promotes the Survival and Progression of Non-Small Cell Lung Cancer.
Constanzo JD, Tang KJ, Rindhe S, Melegari M, Liu H, Tang X, Rodriguez-Canales J, Wistuba I, Scaglioni PP. Constanzo JD, et al. Neoplasia. 2016 May;18(5):282-293. doi: 10.1016/j.neo.2016.03.003. Epub 2016 Apr 16. Neoplasia. 2016. PMID: 27237320 Free PMC article. - A systems-level analysis of the mutually antagonistic roles of RKIP and BACH1 in dynamics of cancer cell plasticity.
Shyam S, Ramu S, Sehgal M, Jolly MK. Shyam S, et al. J R Soc Interface. 2023 Nov;20(208):20230389. doi: 10.1098/rsif.2023.0389. Epub 2023 Nov 15. J R Soc Interface. 2023. PMID: 37963558 Free PMC article. - How cancer cells dictate their microenvironment: present roles of extracellular vesicles.
Naito Y, Yoshioka Y, Yamamoto Y, Ochiya T. Naito Y, et al. Cell Mol Life Sci. 2017 Feb;74(4):697-713. doi: 10.1007/s00018-016-2346-3. Epub 2016 Aug 31. Cell Mol Life Sci. 2017. PMID: 27582126 Free PMC article. Review.
References
- Patnaik SK, Kannisto E, Knudsen S, Yendamuri S. Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res. 2010;70:36–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- T32 CA009299-32/CA/NCI NIH HHS/United States
- 5-R01-CA168484-04/CA/NCI NIH HHS/United States
- 5-R01-CA132608-03/CA/NCI NIH HHS/United States
- P30 CA076292/CA/NCI NIH HHS/United States
- R01 CA132608/CA/NCI NIH HHS/United States
- T32 CA009299/CA/NCI NIH HHS/United States
- 5-P50-CA70907-12 PP-3/CA/NCI NIH HHS/United States
- K08-CA151651/CA/NCI NIH HHS/United States
- R01 CA168484/CA/NCI NIH HHS/United States
- P30CA016672/CA/NCI NIH HHS/United States
- 5-P50-CA70907-12 PP-3B/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- P50 CA070907/CA/NCI NIH HHS/United States
- K08 CA151651/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials