Bacterial resistance to leucyl-tRNA synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections - PubMed (original) (raw)
Clinical Trial
doi: 10.1128/AAC.03774-14. Epub 2014 Oct 27.
Aaron T Spivak 2, Karen Ingraham 1, Sharon Min 1, David J Holmes 1, Charles Jakielaszek 1, Stephen Rittenhouse 1, Alan L Kwan 3, George P Livi 3, Ganesh Sathe 3, Elizabeth Thomas 3, Stephanie Van Horn 3, Linda A Miller 4, Monique Twynholm 5, John Tomayko 4, Marybeth Dalessandro 4, Madelyn Caltabiano 4, Nicole E Scangarella-Oman 4, James R Brown 6
Affiliations
- PMID: 25348524
- PMCID: PMC4291364
- DOI: 10.1128/AAC.03774-14
Clinical Trial
Bacterial resistance to leucyl-tRNA synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections
Karen O'Dwyer et al. Antimicrob Agents Chemother. 2015 Jan.
Abstract
GSK2251052, a novel leucyl-tRNA synthetase (LeuRS) inhibitor, was in development for the treatment of infections caused by multidrug-resistant Gram-negative pathogens. In a phase II study (study LRS114688) evaluating the efficacy of GSK2251052 in complicated urinary tract infections, resistance developed very rapidly in 3 of 14 subjects enrolled, with ≥32-fold increases in the GSK2251052 MIC of the infecting pathogen being detected. A fourth subject did not exhibit the development of resistance in the baseline pathogen but posttherapy did present with a different pathogen resistant to GSK2251052. Whole-genome DNA sequencing of Escherichia coli isolates collected longitudinally from two study LRS114688 subjects confirmed that GSK2251052 resistance was due to specific mutations, selected on the first day of therapy, in the LeuRS editing domain. Phylogenetic analysis strongly suggested that resistant Escherichia coli isolates resulted from clonal expansion of baseline susceptible strains. This resistance development likely resulted from the confluence of multiple factors, of which only some can be assessed preclinically. Our study shows the challenges of developing antibiotics and the importance of clinical studies to evaluate their effect on disease pathogenesis. (These studies have been registered at ClinicalTrials.gov under registration no. NCT01381549 for the study of complicated urinary tract infections and registration no. NCT01381562 for the study of complicated intra-abdominal infections.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
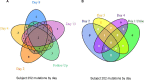
FIG 1
Venn diagram showing the variation in nonsynonymous SNVs in protein-coding genes relative to the sequence of the genome at baseline day 1 for subject 252 (A) and subject 202 (B). Variants in prophage, transposase, and hypothetical genes were excluded from the figure to focus on SNVs in genes with a functional annotation.
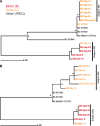
FIG 2
Phylogenetic trees of E. coli strains based on published concatenated nucleotide sequences of representative E. coli isolates causing urinary tract infection. (A) Phylogeny of MLST genes based on the alignment of 9,018 nt. (B) Phylogeny of loci with SNVs based on the alignment of 8,637 nt derived from whole-genome comparisons of day 1 isolates from subjects 202 and 252 to those genomes from the respective subsequent days. Trees were reconstructed using both bootstrap neighbor-joining (shown) and Bayesian methods. Branch points marked with an asterisk were supported in more than 80% of 1,000 bootstrap replications and by a Bayesian posterior probability of ≤0.01. The location of E. coli CFT073, used for gene annotation mapping, is also indicated.
Similar articles
- In Vitro and In Vivo Activities of DS86760016, a Novel Leucyl-tRNA Synthetase Inhibitor for Gram-Negative Pathogens.
Purnapatre KP, Rao M, Pandya M, Khanna A, Chaira T, Bambal R, Upadhyay DJ, Masuda N. Purnapatre KP, et al. Antimicrob Agents Chemother. 2018 Mar 27;62(4):e01987-17. doi: 10.1128/AAC.01987-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29437618 Free PMC article. - Comparative in vitro activities of GSK2251052, a novel boron-containing leucyl-tRNA synthetase inhibitor, against 916 anaerobic organisms.
Goldstein EJ, Citron DM, Tyrrell KL, Merriam CV. Goldstein EJ, et al. Antimicrob Agents Chemother. 2013 May;57(5):2401-4. doi: 10.1128/AAC.02580-12. Epub 2013 Mar 4. Antimicrob Agents Chemother. 2013. PMID: 23459482 Free PMC article. - DS86760016, a Leucyl-tRNA Synthetase Inhibitor with Activity against Pseudomonas aeruginosa.
Kumar M, Rao M, Purnapatre KP, Barman TK, Joshi V, Kashyap A, Chaira T, Bambal RB, Pandya M, Khodor SA, Upadhyay DJ, Masuda N. Kumar M, et al. Antimicrob Agents Chemother. 2019 Mar 27;63(4):e02122-18. doi: 10.1128/AAC.02122-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30670430 Free PMC article.
Cited by
- Spontaneous Selection of Cryptosporidium Drug Resistance in a Calf Model of Infection.
Hasan MM, Stebbins EE, Choy RKM, Gillespie JR, de Hostos EL, Miller P, Mushtaq A, Ranade RM, Teixeira JE, Verlinde CLMJ, Sateriale A, Zhang Z, Osbourn DM, Griggs DW, Fan E, Buckner FS, Huston CD. Hasan MM, et al. Antimicrob Agents Chemother. 2021 May 18;65(6):e00023-21. doi: 10.1128/AAC.00023-21. Print 2021 May 18. Antimicrob Agents Chemother. 2021. PMID: 33753338 Free PMC article. - In Vitro Resistance-Predicting Studies and In Vitro Resistance-Related Parameters-A Hit-to-Lead Perspective.
Krajewska J, Tyski S, Laudy AE. Krajewska J, et al. Pharmaceuticals (Basel). 2024 Aug 15;17(8):1068. doi: 10.3390/ph17081068. Pharmaceuticals (Basel). 2024. PMID: 39204172 Free PMC article. Review. - Antibiotics in the clinical pipeline in October 2019.
Butler MS, Paterson DL. Butler MS, et al. J Antibiot (Tokyo). 2020 Jun;73(6):329-364. doi: 10.1038/s41429-020-0291-8. Epub 2020 Mar 10. J Antibiot (Tokyo). 2020. PMID: 32152527 Free PMC article. Review. - A Polymorphism in leuS Confers Reduced Susceptibility to GSK2251052 in a Clinical Isolate of Staphylococcus aureus.
Gupta A, Monteferrante C, Rasina D, Leitis G, Randall CP, Tomlinson JH, Jirgensons A, Goessens WH, Hays JP, O'Neill AJ. Gupta A, et al. Antimicrob Agents Chemother. 2016 Apr 22;60(5):3219-21. doi: 10.1128/AAC.02940-15. Print 2016 May. Antimicrob Agents Chemother. 2016. PMID: 26976861 Free PMC article. - Antibiotics in the clinical pipeline as of December 2022.
Butler MS, Henderson IR, Capon RJ, Blaskovich MAT. Butler MS, et al. J Antibiot (Tokyo). 2023 Aug;76(8):431-473. doi: 10.1038/s41429-023-00629-8. Epub 2023 Jun 8. J Antibiot (Tokyo). 2023. PMID: 37291465 Free PMC article. Review.
References
- Roberts RR, Hota B, Ahmad I, Scott RD, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 49:1175–1184. doi:10.1086/605630. - DOI - PubMed
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi:10.1086/591861. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases