Simian hemorrhagic fever virus cell entry is dependent on CD163 and uses a clathrin-mediated endocytosis-like pathway - PubMed (original) (raw)
doi: 10.1128/JVI.02697-14. Epub 2014 Oct 29.
Elena N Postnikova 1, John G Bernbaum 1, Shu Qìng Yú 1, Steven Mazur 1, Nicole M Deiuliis 1, Sheli R Radoshitzky 2, Matthew G Lackemeyer 1, Adam McCluskey 3, Phillip J Robinson 4, Volker Haucke 5, Victoria Wahl-Jensen 1, Adam L Bailey 6, Michael Lauck 6, Thomas C Friedrich 6, David H O'Connor 6, Tony L Goldberg 6, Peter B Jahrling 1, Jens H Kuhn 7
Affiliations
- PMID: 25355889
- PMCID: PMC4301170
- DOI: 10.1128/JVI.02697-14
Simian hemorrhagic fever virus cell entry is dependent on CD163 and uses a clathrin-mediated endocytosis-like pathway
Yíngyún Caì et al. J Virol. 2015 Jan.
Abstract
Simian hemorrhagic fever virus (SHFV) causes a severe and almost uniformly fatal viral hemorrhagic fever in Asian macaques but is thought to be nonpathogenic for humans. To date, the SHFV life cycle is almost completely uncharacterized on the molecular level. Here, we describe the first steps of the SHFV life cycle. Our experiments indicate that SHFV enters target cells by low-pH-dependent endocytosis. Dynamin inhibitors, chlorpromazine, methyl-β-cyclodextrin, chloroquine, and concanamycin A dramatically reduced SHFV entry efficiency, whereas the macropinocytosis inhibitors EIPA, blebbistatin, and wortmannin and the caveolin-mediated endocytosis inhibitors nystatin and filipin III had no effect. Furthermore, overexpression and knockout study and electron microscopy results indicate that SHFV entry occurs by a dynamin-dependent clathrin-mediated endocytosis-like pathway. Experiments utilizing latrunculin B, cytochalasin B, and cytochalasin D indicate that SHFV does not hijack the actin polymerization pathway. Treatment of target cells with proteases (proteinase K, papain, α-chymotrypsin, and trypsin) abrogated entry, indicating that the SHFV cell surface receptor is a protein. Phospholipases A2 and D had no effect on SHFV entry. Finally, treatment of cells with antibodies targeting CD163, a cell surface molecule identified as an entry factor for the SHFV-related porcine reproductive and respiratory syndrome virus, diminished SHFV replication, identifying CD163 as an important SHFV entry component.
Importance: Simian hemorrhagic fever virus (SHFV) causes highly lethal disease in Asian macaques resembling human illness caused by Ebola or Lassa virus. However, little is known about SHFV's ecology and molecular biology and the mechanism by which it causes disease. The results of this study shed light on how SHFV enters its target cells. Using electron microscopy and inhibitors for various cellular pathways, we demonstrate that SHFV invades cells by low-pH-dependent, actin-independent endocytosis, likely with the help of a cellular surface protein.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
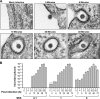
FIG 1
SHFV particles rapidly enter target cells. (A) Time-dependent transmission electron micrographs of SHFV particle entry into MA-104 cells. Shown are representative images of two independent experiments. (B) Growth kinetics of SHFV at various MOIs in MA-104 cells as determined by plaque assay. The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
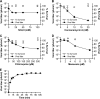
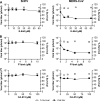
FIG 2
SHFV particles enter target cells in a low-pH-dependent manner. (A to D) Effects of pretreatment of MA-104 cells with increasing concentrations of inhibitors affecting pH on SHFV particle yield, as determined by plaque assay. (E) Time-of-addition experiment using chloroquine at a fixed concentration of 150 μM. The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
FIG 3
SHFV particles enter cells using a clathrin-mediated endocytosis-like pathway. (A to D) Effects of pretreatment of MA-104 cells with increasing concentrations of inhibitors of clathrin-mediated endocytosis on SHFV particle yield as determined by plaque assay. (E) Time-of-addition experiment testing the effects of Dyngo-4a at the same concentration but at different points after cell exposure to SHFV particles. (F) Effect of overexpression of wild-type dynamin 1 or a dominant-negative mutant thereof in MA-104 cells on SHFV progeny production. (G) Evaluation of clathrin HC expression in MA-104 cells treated with clathrin HC-specific gRNAs or control gRNA by Western blotting. (H) Effects of gRNA treatment of MA-104 cells on SHFV progeny production. The error bars indicate the standard deviations of triplicate samples of one of two independent experiments. <, measurement below the threshold of detection (20 PFU/ml); *, P < 0.05 (Student's t test).
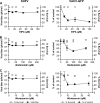
FIG 4
SHFV particles do not enter cells by macropinocytosis. (A to C) Effects of pretreatment of MA-104 cells with increasing concentrations of inhibitors of macropinocytosis on the SHFV viral titer, as determined by plaque assay (left), or on the percentage of VACV-GFP-infected cells, as determined by high-content imaging (right). The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
FIG 5
SHFV cell entry is independent of actin polymerization. (A to C) (Left) Effects of pretreatment of MA-104 cells with increasing concentrations of inhibitors of actin polymerization on SHFV particle yield, as determined by plaque assay. (Right) Immunofluorescence images of the MA-104 cells showing the disruption of actin networks by the inhibitors using Alexa 594-phalloidin staining. (D) Alexa 594-phalloidin staining of untreated MA-104 cells treated with dimethyl sulfoxide (DMSO). The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
FIG 6
SHFV particles do not enter cells by caveola-mediated endocytosis. (A and B) Effects of pretreatment of MA-104 cells with increasing concentrations of inhibitors of caveola-mediated endocytosis on SHFV particle yield, as determined by plaque assay. (C and D) Effects of pretreatment of MA-104 cells with increasing concentrations of the same inhibitors on RVFV infection, as determined by IFA. (E) Influence of cholesterol depletion on SHFV particle yield. Cells were treated with 5 or 10 mM methyl-β-cyclodextrin (MβCD) and infected with SHFV. The particle yield was determined by plaque assay. Alternatively, the cells were treated with MβCD, and exogenous soluble cholesterol was added to reverse the effect of MβCD. The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
FIG 7
Cathepsins L and B do not play a role in SHFV cell entry and replication. (A to C) Effects of pretreatment of MA-104 cells with increasing concentrations of cathepsin inhibitors on SHFV and MERS-CoV (positive control) particle yield, as determined by plaque assay. MA-104 cells were pretreated with E-64d (cathepsin L and B inhibitor), FYdmk (cathepsin L inhibitor), or CA-074 (cathepsin B inhibitor) for 4 h and then infected with SHFV or MERS-CoV at an MOI of 5. The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
FIG 8
SHFV particles use a proteinaceous cell surface receptor to gain entry into target cells. (A to D) Effects of pretreatment of MA-104 cells with increasing concentrations of proteases on SHFV particle yield, as determined by plaque assay. (E and F) Effects of pretreatment of MA-104 cells with increasing concentrations of phospholipase A2 or D on SHFV particle yield. The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
FIG 9
CD163 is a crucial SHFV cell entry factor. (A and B) Effects of incubation of MA-104 or MARC-145 cells with increasing concentrations of human anti-CD163 or control antibody on SHFV and PRRSV particle yield, as determined by plaque assay. The error bars indicate the standard deviations of triplicate samples from one of two independent experiments.
Similar articles
- Divergent Simian Arteriviruses Cause Simian Hemorrhagic Fever of Differing Severities in Macaques.
Wahl-Jensen V, Johnson JC, Lauck M, Weinfurter JT, Moncla LH, Weiler AM, Charlier O, Rojas O, Byrum R, Ragland DR, Huzella L, Zommer E, Cohen M, Bernbaum JG, Caì Y, Sanford HB, Mazur S, Johnson RF, Qin J, Palacios GF, Bailey AL, Jahrling PB, Goldberg TL, O'Connor DH, Friedrich TC, Kuhn JH. Wahl-Jensen V, et al. mBio. 2016 Feb 23;7(1):e02009-15. doi: 10.1128/mBio.02009-15. mBio. 2016. PMID: 26908578 Free PMC article. - Within-Host Evolution of Simian Arteriviruses in Crab-Eating Macaques.
Moncla LH, Weiler AM, Barry G, Weinfurter JT, Dinis JM, Charlier O, Lauck M, Bailey AL, Wahl-Jensen V, Nelson CW, Johnson JC, Caì Y, Goldberg TL, O'Connor DH, Jahrling PB, Kuhn JH, Friedrich TC. Moncla LH, et al. J Virol. 2017 Jan 31;91(4):e02231-16. doi: 10.1128/JVI.02231-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974564 Free PMC article. - Primate hemorrhagic fever-causing arteriviruses are poised for spillover to humans.
Warren CJ, Yu S, Peters DK, Barbachano-Guerrero A, Yang Q, Burris BL, Worwa G, Huang IC, Wilkerson GK, Goldberg TL, Kuhn JH, Sawyer SL. Warren CJ, et al. Cell. 2022 Oct 13;185(21):3980-3991.e18. doi: 10.1016/j.cell.2022.09.022. Epub 2022 Sep 30. Cell. 2022. PMID: 36182704 Free PMC article. - Simian hemorrhagic fever virus: Recent advances.
Brinton MA, Di H, Vatter HA. Brinton MA, et al. Virus Res. 2015 Apr 16;202:112-9. doi: 10.1016/j.virusres.2014.11.024. Epub 2014 Nov 29. Virus Res. 2015. PMID: 25455336 Free PMC article. Review. - PRRS virus receptors and their role for pathogenesis.
Zhang Q, Yoo D. Zhang Q, et al. Vet Microbiol. 2015 Jun 12;177(3-4):229-41. doi: 10.1016/j.vetmic.2015.04.002. Epub 2015 Apr 13. Vet Microbiol. 2015. PMID: 25912022 Review.
Cited by
- Specific Detection of Two Divergent Simian Arteriviruses Using RNAscope In Situ Hybridization.
Yú SQ, Caì Y, Lyons C, Johnson RF, Postnikova E, Mazur S, Johnson JC, Radoshitzky SR, Bailey AL, Lauck M, Goldberg TL, O'Connor DH, Jahrling PB, Friedrich TC, Kuhn JH. Yú SQ, et al. PLoS One. 2016 Mar 10;11(3):e0151313. doi: 10.1371/journal.pone.0151313. eCollection 2016. PLoS One. 2016. PMID: 26963736 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - Porcine Reproductive and Respiratory Syndrome Virus strains with Higher Virulence Cause Marked Protein Profile Changes in MARC-145 Cells.
Chen Z, Liu S, Zhang S, Zhang Y, Yu J, Sun W, Chen L, Du Y, Wang J, Li Y, Wu J. Chen Z, et al. Sci Rep. 2018 Oct 9;8(1):15000. doi: 10.1038/s41598-018-32984-0. Sci Rep. 2018. PMID: 30302013 Free PMC article. - Clinical Characterization of Host Response to Simian Hemorrhagic Fever Virus Infection in Permissive and Refractory Hosts: A Model for Determining Mechanisms of VHF Pathogenesis.
Cornish JP, Moore IN, Perry DL, Lara A, Minai M, Promeneur D, Hagen KR, Virtaneva K, Paneru M, Buechler CR, O'Connor DH, Bailey AL, Cooper K, Mazur S, Bernbaum JG, Pettitt J, Jahrling PB, Kuhn JH, Johnson RF. Cornish JP, et al. Viruses. 2019 Jan 15;11(1):67. doi: 10.3390/v11010067. Viruses. 2019. PMID: 30650570 Free PMC article. - Reappraising host cellular factors involved in attachment and entry to develop antiviral strategies against porcine reproductive and respiratory syndrome virus.
Li R, Qiao S, Zhang G. Li R, et al. Front Microbiol. 2022 Jul 26;13:975610. doi: 10.3389/fmicb.2022.975610. eCollection 2022. Front Microbiol. 2022. PMID: 35958155 Free PMC article. Review.
References
- Faaberg KS, Balasuriya UB, Brinton MA, Gorbalenya AE, Leung FC-C, Nauwynck H, Snijder EJ, Stadejek T, Yang H, Yoo D. 2011. Family Arteriviridae, p 796–805. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom.
- Lauck M, Hyeroba D, Tumukunde A, Weny G, Lank SM, Chapman CA, O'Connor DH, Friedrich TC, Goldberg TL. 2011. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS One 6:e19056. doi:10.1371/journal.pone.0019056. - DOI - PMC - PubMed
- Bailey AL, Lauck M, Weiler A, Sibley SD, Dinis JM, Bergman Z, Nelson CW, Correll M, Gleicher M, Hyeroba D, Tumukunde A, Weny G, Chapman C, Kuhn JH, Hughes AL, Friedrich TC, Goldberg TL, O'Connor DH. 2014. High genetic diversity and adaptive potential of two simian hemorrhagic fever viruses in a wild primate population. PLoS One 9:e90714. doi:10.1371/journal.pone.0090714. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN272200700016I/AI/NIAID NIH HHS/United States
- P51 OD011106/OD/NIH HHS/United States
- T32 GM008692/GM/NIGMS NIH HHS/United States
- T32 GM081061/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials