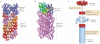SMOCs: supramolecular organizing centres that control innate immunity - PubMed (original) (raw)
Review
SMOCs: supramolecular organizing centres that control innate immunity
Jonathan C Kagan et al. Nat Rev Immunol. 2014 Dec.
Abstract
The diverse receptor families of the innate immune system activate signal transduction pathways that are important for host defence, but common themes to explain the operation of these pathways remain undefined. In this Opinion article, we propose--on the basis of recent structural and cell biological studies--the concept of supramolecular organizing centres (SMOCs) as location-specific higher-order signalling complexes in which increased local concentrations of signalling components promote the intrinsically weak allosteric interactions that are required for enzyme activation. We suggest that SMOCs are assembled on various membrane-bound organelles or other intracellular sites, which may assist signal amplification to reach a response threshold and potentially define the specificity of cellular responses that are induced in response to infectious and non-infectious insults.
Figures
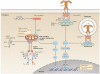
Figure 1. SMOC formation for TLRs, RLRs and NLRs
Depicted are the best-studied supramolecular organizing centres (SMOCs), including the ligands and regulatory proteins that promote their assembly, and the downstream biological activities induced by these protein complexes. The figure does not show the exact stoichiometry of the protein components in each signalling complex. Binding of lipopolysaccharide (not shown) activates Toll-like receptor 4 (TLR4), leading to assembly of a Myddosome on the plasma membrane. By contrast, unmethylated CpG-containing DNA oligonucleotides (not shown) promote TLR9 to assemble an endosomal Myddosome. The TLR-specific sorting adaptor Toll/IL-1R domain-containing adaptor protein (TIRAP) facilitates Myddosome assembly. TIRAP has an amino-terminal lipid-binding domain that interacts promiscuously with acidic phosphoinositides and phosphatidylserine. For example, TIRAP is depicted as binding phosphatidylinositol-3-phosphate (PtdIns3P) and phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) on the endosomal membrane and the plasma membrane, respectively. Binding of cardiolipin, which translocates to the outer mitochondrial membrane upon mitochondrial dysfunction, relieves the autoinhibited state of NOD-, LRR- and pyrin domain-containing 3 (NLRP3). This in turn may promote NLRP3 inflammasome assembly through downstream pyrin domain (PYD)–PYD and caspase activation and recruitment domain (CARD)–CARD interactions. Activation of RIG-I-like receptors — retinoic acid-inducible gene I (RIG-I) or melanoma differentiation-associated protein 5 (MDA5) — leads to the formation of higher-order oligomers of mitochondrial antiviral signalling protein (MAVS). IFN, interferon; IκBα, NF-κB inhibitor-α; IL, interleukin; IRAK, IL-1 receptor-associated kinase; IRF, IFN-regulatory factor; LRR, leucine-rich repeat; MYD88, myeloid differentiation primary response protein 88; NOD, nucleotide-binding oligomerization domain; NF-κB, nuclear factor-κB; TAB1, TAK1-binding protein 1; TAK1, TGFβ-associated kinase 1; TRAF, tumour necrosis factor receptor-associated factor.
Figure 2. Structures of SMOCs that are formed by the mechanism of nucleated polymerization
a | A ribbon diagram of the electron cryomicroscopic structure of polymerized ASC pyrin domain (ASCPYD) filaments in inflammasomes (shown in red, purple and orange for each of the three helical strands), in complex with polymerized absent in melanoma 2 PYD (AIM2PYD) filaments (shown in blue) formed upon double-stranded DNA (dsDNA) stimulation. b | A ribbon diagram of the electron cryomicroscopic structure of polymerized mitochondrial antiviral signalling protein caspase activation and recruitment domain (MAVSCARD) in the RIG-I-like receptor (RLR) pathway (shown in purple), in complex with a retinoic acid-inducible gene I (RIG-I) double CARD (RIG-I2CARD) tetramer (shown in blue, cyan, green and yellow) upon viral RNA stimulation. c | Proposed mechanism of nucleated polymerization for the formation of ASCPYD and MAVSCARD SMOCs. Receptors (for example, AIM2 and RIG-I) are shown in red wedges for monomers and in red disks for oligomerized forms. Adaptors (for example, ASC and MAVS) are shown in blue wedges for monomers and in blue cylinders for filaments.
Similar articles
- Toll-like Receptors and the Control of Immunity.
Fitzgerald KA, Kagan JC. Fitzgerald KA, et al. Cell. 2020 Mar 19;180(6):1044-1066. doi: 10.1016/j.cell.2020.02.041. Epub 2020 Mar 11. Cell. 2020. PMID: 32164908 Free PMC article. Review. - Supramolecular organizing centers (SMOCs) as signaling machines in innate immune activation.
Qiao Q, Wu H. Qiao Q, et al. Sci China Life Sci. 2015 Nov;58(11):1067-72. doi: 10.1007/s11427-015-4951-z. Epub 2015 Oct 28. Sci China Life Sci. 2015. PMID: 26511517 Free PMC article. Review. - Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease.
Cao X. Cao X. Nat Rev Immunol. 2016 Jan;16(1):35-50. doi: 10.1038/nri.2015.8. Nat Rev Immunol. 2016. PMID: 26711677 Review. - Regulation of pattern recognition receptor signalling in plants.
Couto D, Zipfel C. Couto D, et al. Nat Rev Immunol. 2016 Sep;16(9):537-52. doi: 10.1038/nri.2016.77. Epub 2016 Aug 1. Nat Rev Immunol. 2016. PMID: 27477127 Review. - Innate Immune Signaling Organelles Display Natural and Programmable Signaling Flexibility.
Tan Y, Kagan JC. Tan Y, et al. Cell. 2019 Apr 4;177(2):384-398.e11. doi: 10.1016/j.cell.2019.01.039. Epub 2019 Mar 7. Cell. 2019. PMID: 30853218 Free PMC article.
Cited by
- Dynamic Protease Activation on a Multimeric Synthetic Protein Scaffold via Adaptable DNA-Based Recruitment Domains.
Mashima T, Rosier BJHM, Oohora K, de Greef TFA, Hayashi T, Brunsveld L. Mashima T, et al. Angew Chem Int Ed Engl. 2021 May 10;60(20):11262-11266. doi: 10.1002/anie.202102160. Epub 2021 Apr 8. Angew Chem Int Ed Engl. 2021. PMID: 33725379 Free PMC article. - Profile of Hao Wu.
Ahmed F. Ahmed F. Proc Natl Acad Sci U S A. 2019 Jan 22;116(4):1078-1080. doi: 10.1073/pnas.1821528116. Epub 2019 Jan 14. Proc Natl Acad Sci U S A. 2019. PMID: 30642964 Free PMC article. No abstract available. - Inflammasomes as regulators of non-infectious disease.
Okin D, Kagan JC. Okin D, et al. Semin Immunol. 2023 Sep;69:101815. doi: 10.1016/j.smim.2023.101815. Epub 2023 Jul 26. Semin Immunol. 2023. PMID: 37506489 Free PMC article. Review. - Toward targeting inflammasomes: insights into their regulation and activation.
Christgen S, Place DE, Kanneganti TD. Christgen S, et al. Cell Res. 2020 Apr;30(4):315-327. doi: 10.1038/s41422-020-0295-8. Epub 2020 Mar 9. Cell Res. 2020. PMID: 32152420 Free PMC article. Review. - Visualization of Myddosome Assembly in Live Cells.
Cao F, Taylor MJ. Cao F, et al. Methods Mol Biol. 2023;2654:231-250. doi: 10.1007/978-1-0716-3135-5_15. Methods Mol Biol. 2023. PMID: 37106186
References
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
- Medzhitov R, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253–258. - PubMed
- Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. - PubMed
- Yamamoto M, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nature Immunol. 2003;4:1144–1150. - PubMed
- Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI093589/AI/NIAID NIH HHS/United States
- R01 AI050872/AI/NIAID NIH HHS/United States
- AI113141-01/AI/NIAID NIH HHS/United States
- R56 AI113141/AI/NIAID NIH HHS/United States
- K99 AI072955/AI/NIAID NIH HHS/United States
- R37 AI050872/AI/NIAID NIH HHS/United States
- AI072955/AI/NIAID NIH HHS/United States
- R56 AI093589/AI/NIAID NIH HHS/United States
- AI093589/AI/NIAID NIH HHS/United States
- R00 AI072955/AI/NIAID NIH HHS/United States
- R01 AI045937/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources