GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia - PubMed (original) (raw)
Comparative Study
. 2015 Jan 1;125(1):56-70.
doi: 10.1182/blood-2014-06-580340. Epub 2014 Oct 30.
Danielle M Townsley 2, Amy P Hsu 3, Diane C Arthur 1, Christa S Zerbe 3, Jennifer Cuellar-Rodriguez 3, Dennis D Hickstein 4, Sergio D Rosenzweig 5, Raul C Braylan 6, Neal S Young 2, Steven M Holland 3, Katherine R Calvo 6
Affiliations
- PMID: 25359990
- PMCID: PMC4281830
- DOI: 10.1182/blood-2014-06-580340
Comparative Study
GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia
Karthik A Ganapathi et al. Blood. 2015.
Abstract
Germ-line GATA2 gene mutations, leading to haploinsufficiency, have been identified in patients with familial myelodysplastic syndrome/acute myeloid leukemia, monocytopenia and mycobacterial infections, Emberger syndrome, and dendritic cell, monocyte, B-, and NK-cell deficiency. GATA2 mutations have also been reported in a minority of patients with congenital neutropenia and aplastic anemia (AA). The bone marrow (BM) from patients with GATA2 deficiency is typically hypocellular, with varying degrees of dysplasia. Distinguishing GATA2 patients from those with AA is critical for selecting appropriate therapy. We compared the BM flow cytometric, morphologic, and cytogenetic features of 28 GATA2 patients with those of 32 patients being evaluated for idiopathic AA. The marrow of GATA2 patients had severely reduced monocytes, B cells, and NK cells; absent hematogones; and inverted CD4:CD8 ratios. Atypical megakaryocytes and abnormal cytogenetics were more common in GATA2 marrows. CD34(+) cells were comparably reduced in GATA2 and AA. Using these criteria, we prospectively identified 4 of 32 patients with suspected AA who had features suspicious for GATA2 mutations, later confirmed by DNA sequencing. Our results show that routine BM flow cytometry, morphology, and cytogenetics in patients who present with cytopenia(s) can identify patients for whom GATA2 sequencing is indicated.
Figures
Figure 1
Peripheral blood indices of GATA2 and AA patients. (A) GATA2 and AA patients have comparable ages, hemoglobin levels, and WBC counts, but GATA2 patients have higher platelet, neutrophil count, and lower lymphocyte count compared with AA patients. (B) GATA2 patients have reduced peripheral blood monocytes, B cells, and NK cells, and relatively increased T cells compared with AA patients.
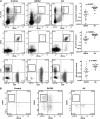
Figure 2
Bone marrow flow cytometric analysis of monocytes and lymphoid subsets in GATA2 and AA patients. (A) GATA2 patients have reduced bone marrow monocytes (CD33+, HLA-DR+), mature B cells (CD19+, CD20+), and NK cells (sCD3−, CD56+) compared with AA patients. (B) A subset of GATA2 patients has atypical CD19−CD56+ plasma cells (gating performed on CD38+, CD138+ positive population), which are not identified in AA patients.
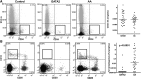
Figure 3
Comparison of T-cell subsets between GATA2 and AA patients. (A) GATA2 patients have relatively increased bone marrow T cells, (B) inverted CD4:CD8 ratios, (C) increased CD3+CD56+, and (D) increased CD3+CD57+ T-cell subsets compared with AA patients.
Figure 4
Comparison of bone marrow precursor cells between GATA2 and AA patients. (A) GATA2 and AA patients have comparable levels of CD34-positive cells. (B) GATA2 patients have absent hematogones compared with AA patients.
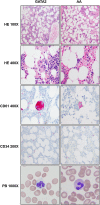
Figure 5
Comparison of peripheral blood and bone marrow morphologic and immunohistochemical features between GATA2 and AA patients. GATA2 patient marrows have greater overall cellularity, increased megakaryocytes with atypical features (highlighted by CD61), but overall similar numbers of CD34-positive cells compared with AA marrows. A subset of GATA2 patients has hypogranular circulating neutrophils.
Similar articles
- Germline GATA2 Mutation and Bone Marrow Failure.
McReynolds LJ, Calvo KR, Holland SM. McReynolds LJ, et al. Hematol Oncol Clin North Am. 2018 Aug;32(4):713-728. doi: 10.1016/j.hoc.2018.04.004. Epub 2018 May 28. Hematol Oncol Clin North Am. 2018. PMID: 30047422 Free PMC article. Review. - Clinical impact of dysplastic changes in acquired aplastic anemia: A systematic study of bone marrow biopsies in children and adults.
Marchesi RF, Velloso EDRP, Garanito MP, Leal AM, Siqueira SAC, Azevedo Neto RS, Rocha V, Zerbini MCN. Marchesi RF, et al. Ann Diagn Pathol. 2020 Apr;45:151459. doi: 10.1016/j.anndiagpath.2019.151459. Epub 2019 Dec 30. Ann Diagn Pathol. 2020. PMID: 32000075 - Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome.
Nováková M, Žaliová M, Suková M, Wlodarski M, Janda A, Froňková E, Campr V, Lejhancová K, Zapletal O, Pospíšilová D, Černá Z, Kuhn T, Švec P, Pelková V, Zemanová Z, Kerndrup G, van den Heuvel-Eibrink M, van der Velden V, Niemeyer C, Kalina T, Trka J, Starý J, Hrušák O, Mejstříková E. Nováková M, et al. Haematologica. 2016 Jun;101(6):707-16. doi: 10.3324/haematol.2015.137711. Epub 2016 Mar 24. Haematologica. 2016. PMID: 27013649 Free PMC article. - Loss of c-Kit and bone marrow failure upon conditional removal of the GATA-2 C-terminal zinc finger domain in adult mice.
Li HS, Jin J, Liang X, Matatall KA, Ma Y, Zhang H, Ullrich SE, King KY, Sun SC, Watowich SS. Li HS, et al. Eur J Haematol. 2016 Sep;97(3):261-70. doi: 10.1111/ejh.12719. Epub 2016 Jan 14. Eur J Haematol. 2016. PMID: 26660446 Free PMC article. - Acquired and germline predisposition to bone marrow failure: Diagnostic features and clinical implications.
Kallen ME, Dulau-Florea A, Wang W, Calvo KR. Kallen ME, et al. Semin Hematol. 2019 Jan;56(1):69-82. doi: 10.1053/j.seminhematol.2018.05.016. Epub 2018 Jun 23. Semin Hematol. 2019. PMID: 30573048 Review.
Cited by
- GATA2 deficiency and hemophagocytic lymphohistiocytosis (HLH): a systematic review of reported cases.
Rukerd MRZ, Mirkamali H, Nakhaie M, Alizadeh SD. Rukerd MRZ, et al. BMC Infect Dis. 2024 Nov 4;24(1):1239. doi: 10.1186/s12879-024-10145-1. BMC Infect Dis. 2024. PMID: 39497062 Free PMC article. - Multidisciplinary approaches to study anaemia with special mention on aplastic anaemia (Review).
Sankar D, Oviya IR. Sankar D, et al. Int J Mol Med. 2024 Nov;54(5):95. doi: 10.3892/ijmm.2024.5419. Epub 2024 Sep 2. Int J Mol Med. 2024. PMID: 39219286 Free PMC article. Review. - MicroRNA dysregulation in myelodysplastic syndromes: implications for diagnosis, prognosis, and therapeutic response.
Micheva ID, Atanasova SA. Micheva ID, et al. Front Oncol. 2024 Aug 2;14:1410656. doi: 10.3389/fonc.2024.1410656. eCollection 2024. Front Oncol. 2024. PMID: 39156702 Free PMC article. Review. - Inborn Errors of Immunity and Cytokine Storm Syndromes.
Reid W, Romberg N. Reid W, et al. Adv Exp Med Biol. 2024;1448:185-207. doi: 10.1007/978-3-031-59815-9_14. Adv Exp Med Biol. 2024. PMID: 39117816 Review. - Malignant progression of preleukemic disorders.
Hall T, Gurbuxani S, Crispino JD. Hall T, et al. Blood. 2024 May 30;143(22):2245-2255. doi: 10.1182/blood.2023020817. Blood. 2024. PMID: 38498034 Review.
References
- Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43(10):929–931. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials