Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE-MS) rather than affinity purification mass spectrometry (AP-MS) - PubMed (original) (raw)
Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE-MS) rather than affinity purification mass spectrometry (AP-MS)
Eva C Keilhauer et al. Mol Cell Proteomics. 2015 Jan.
Abstract
Protein-protein interactions are fundamental to the understanding of biological processes. Affinity purification coupled to mass spectrometry (AP-MS) is one of the most promising methods for their investigation. Previously, complexes were purified as much as possible, frequently followed by identification of individual gel bands. However, todays mass spectrometers are highly sensitive, and powerful quantitative proteomics strategies are available to distinguish true interactors from background binders. Here we describe a high performance affinity enrichment-mass spectrometry method for investigating protein-protein interactions, in which no attempt at purifying complexes to homogeneity is made. Instead, we developed analysis methods that take advantage of specific enrichment of interactors in the context of a large amount of unspecific background binders. We perform single-step affinity enrichment of endogenously expressed GFP-tagged proteins and their interactors in budding yeast, followed by single-run, intensity-based label-free quantitative LC-MS/MS analysis. Each pull-down contains around 2000 background binders, which are reinterpreted from troubling contaminants to crucial elements in a novel data analysis strategy. First the background serves for accurate normalization. Second, interacting proteins are not identified by comparison to a single untagged control strain, but instead to the other tagged strains. Third, potential interactors are further validated by their intensity profiles across all samples. We demonstrate the power of our AE-MS method using several well-known and challenging yeast complexes of various abundances. AE-MS is not only highly efficient and robust, but also cost effective, broadly applicable, and can be performed in any laboratory with access to high-resolution mass spectrometers.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Fig. 1.
Schematic representation of the AE-MS workflow. A, Endogenously expressed GFP-tagged proteins are extracted from yeast cells using mild, nondenaturing conditions. B = Bait, I = Interactor, U = Unspecific binder. B, Bait protein and specific interactors are enriched in a single-step immunoprecipitation using anti-GFP antibodies. Subsequently, bound proteins are digested into peptides. C, The peptide mixture is analyzed by single-shot liquid chromatography tandem mass spectrometry (LC-MS/MS) on an Orbitrap instrument. D, Raw data are processed with MaxQuant to identify and quantify proteins. The resulting label-free quantification (LFQ) intensity matrix is the basis for all downstream data analysis aimed at identifying interactors of the tagged bait proteins.
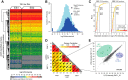
Fig. 2.
The proteomic nature of the background in AE-MS. A, Heatmap of the LFQ intensities of all proteins identified in two experimental series (ES1 and ES2). Hierarchical row clustering was performed on the logarithmized LFQ intensities of more than 2000 quantified prey proteins in the 196 pull-downs, without data imputation. B, Histogram of the copy numbers of all proteins quantified in our pull-downs compared with the entire yeast proteome as in Kulak et al. C, The standard deviation of the LFQ intensity profile for each identified protein was calculated after imputing missing values. Proteins were then ranked according to the standard deviation of their profile. About 70% of detected proteins show a profile varying less than 1 log2 LFQ intensity unit and about 90% vary less than 1.5 log2 LFQ intensity units. D, Comparison of the control strain pHIS3-GFP with the two tagged strains SET1-GFP and PAF1-GFP; all measured in triplicates. The matrix of 36 correlation plots reveals very high correlations between LFQ intensities within triplicates (Pearson correlation coefficient > 0.977 for all strains). The correlation between different strains is always higher than 0.935. Average correlation of the corresponding nine comparisons were: SET1-GFP to PAF1-GFP 0.946, SET1-GFP to control strain 0.938, and PAF1-GFP to control strain 0.945. E, Zoom into the SET1-GFP_01 versus PAF1-GFP_01 correlation plot. The majority of proteins are detected at very similar LFQ intensities in both pull downs. The proteins that differ the most between the two strains are the members of the two targeted complexes highlighted in color.
Fig. 3.
Comparing to unrelated tagged strains. All pull-downs in this figure were measured in quadruplicates. Cut-off lines were those of ES2 (see Experimental Procedures). Red dots represent members of the SKI complex and blue dots represent members of the condensin complex. A, Comparison of the control strain pHIS3-GFP against its parental strain BY4741. B, Classic comparison of a tagged strain against an untagged control strain, in this case SKI2-GFP against pHIS3-GFP. C, SKI2-GFP compared with an unrelated tagged strain, SMC2-GFP. D, SKI2-GFP compared with 8 × pHIS3-GFP in quadruplicate (= 32 control pull-downs). E, SKI2-GFP compared with eight unrelated tagged strains in quadruplicate (APC1-GFP, CAF1-GFP, CCR4-GFP, PAF1-GFP, PEP5-GFP, SMC1-GFP, SMC2-GFP, and SNF4-GFP = 32 control pull-downs). F, SKI2-GFP compared with its bait specific control group (BSCG) consisting of all other pull-downs in the data set except for the SKI3 quadruplicate (= 116 control pull-downs).
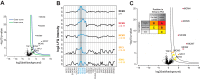
Fig. 4.
Classification of interactors. Proteins are classified as interactors according to their position in the volcano plot and according to their correlation to the corresponding bait protein. A, Volcano Plot. Potential interactors are preclassified according to their position in the volcano plot into “mildly enriched” (between the two curves) and “strongly enriched” (outside the blue curve) proteins B, Intensity profile analysis of some enriched proteins from the volcano plot in A. From top to bottom: intensity profile of MCM4 (the bait protein), MCM6, and MCM3 (true interactors), and SFC1 and SDH3 (false positives) with the according calculated correlation to the profile of MCM4. C, Same volcano plot as in A, but with classification of interactors. Insert: Enrichment, reproducibility and correlation are combined to score interactors into interactor confidence classes A, B and C. Proteins between the cutoff curves with a low correlation (lower than 0.1) were not considered at all. Both proteins between the cutoff curves with a medium correlation (between 0.1 and the series-specific correlation cutoff) and proteins outside the outer cutoff curve with a low correlation (lower than 0.1) were assigned to class C (noninteractors). Proteins between the cutoff curves with a high correlation (higher than the series-specific correlation cutoff) as well as proteins outside the outer cutoff curve with a medium correlation were assigned to class B (lower confidence interactors). Proteins outside the outer cutoff curve with a high correlation were assigned to class A (high confidence interactors).
Fig. 5.
Correlation analysis and mutually exclusive binding. A, Schematic representation of the three alternate SNF1 protein kinase complexes. B, Volcano plot of GAL83 compared with its bait-specific control group (BSCG). C, Volcano plot of SNF4 compared with its BSCG. D, Intensity profiles of the gamma subunit SNF4, the alpha subunit SNF1, and the three alternate beta subunits GAL83, SIP1, and SIP2 as well as their correlation to the bait SNF4.
Similar articles
- Single-Step Affinity Purification (ssAP) and Mass Spectrometry of Macromolecular Complexes in the Yeast S. cerevisiae.
Trahan C, Aguilar LC, Oeffinger M. Trahan C, et al. Methods Mol Biol. 2016;1361:265-87. doi: 10.1007/978-1-4939-3079-1_15. Methods Mol Biol. 2016. PMID: 26483027 - Identification of protein complexes with quantitative proteomics in S. cerevisiae.
Chao JT, Foster LJ, Loewen CJ. Chao JT, et al. J Vis Exp. 2009 Mar 4;(25):1225. doi: 10.3791/1225. J Vis Exp. 2009. PMID: 19262458 Free PMC article. - Resolving protein interactions and complexes by affinity purification followed by label-based quantitative mass spectrometry.
Trinkle-Mulcahy L. Trinkle-Mulcahy L. Proteomics. 2012 May;12(10):1623-38. doi: 10.1002/pmic.201100438. Proteomics. 2012. PMID: 22610586 Review. - Proteomics-Based Analysis of Protein Complexes in Pluripotent Stem Cells and Cancer Biology.
Sudhir PR, Chen CH. Sudhir PR, et al. Int J Mol Sci. 2016 Mar 22;17(3):432. doi: 10.3390/ijms17030432. Int J Mol Sci. 2016. PMID: 27011181 Free PMC article. Review.
Cited by
- The social and structural architecture of the yeast protein interactome.
Michaelis AC, Brunner AD, Zwiebel M, Meier F, Strauss MT, Bludau I, Mann M. Michaelis AC, et al. Nature. 2023 Dec;624(7990):192-200. doi: 10.1038/s41586-023-06739-5. Epub 2023 Nov 15. Nature. 2023. PMID: 37968396 Free PMC article. - Evolutionary origins and interactomes of human, young microproteins and small peptides translated from short open reading frames.
Sandmann CL, Schulz JF, Ruiz-Orera J, Kirchner M, Ziehm M, Adami E, Marczenke M, Christ A, Liebe N, Greiner J, Schoenenberger A, Muecke MB, Liang N, Moritz RL, Sun Z, Deutsch EW, Gotthardt M, Mudge JM, Prensner JR, Willnow TE, Mertins P, van Heesch S, Hubner N. Sandmann CL, et al. Mol Cell. 2023 Mar 16;83(6):994-1011.e18. doi: 10.1016/j.molcel.2023.01.023. Epub 2023 Feb 17. Mol Cell. 2023. PMID: 36806354 Free PMC article. - Native holdup (nHU) to measure binding affinities from cell extracts.
Zambo B, Morlet B, Negroni L, Trave G, Gogl G. Zambo B, et al. Sci Adv. 2022 Dec 21;8(51):eade3828. doi: 10.1126/sciadv.ade3828. Epub 2022 Dec 21. Sci Adv. 2022. PMID: 36542723 Free PMC article. - Mono-Sized Anion-Exchange Magnetic Microspheres for Protein Adsorption.
Wang Z, Wang W, Meng Z, Xue M. Wang Z, et al. Int J Mol Sci. 2022 Apr 29;23(9):4963. doi: 10.3390/ijms23094963. Int J Mol Sci. 2022. PMID: 35563351 Free PMC article. - Systematic assessment of antibody selectivity in plasma based on a resource of enrichment profiles.
Fredolini C, Byström S, Sanchez-Rivera L, Ioannou M, Tamburro D, Pontén F, Branca RM, Nilsson P, Lehtiö J, Schwenk JM. Fredolini C, et al. Sci Rep. 2019 Jun 6;9(1):8324. doi: 10.1038/s41598-019-43552-5. Sci Rep. 2019. PMID: 31171813 Free PMC article.
References
- Gingras A. C., Gstaiger M., Raught B., Aebersold R. (2007) Analysis of protein complexes using mass spectrometry. Nat. Rev. Mol. Cell Biol. 8, 645–654 - PubMed
- Oeffinger M. (2012) Two steps forward–one step back: advances in affinity purification mass spectrometry of macromolecular complexes. Proteomics 12, 1591–1608 - PubMed
- Gavin A. C., Maeda K., Kuhner S. (2011) Recent advances in charting protein–protein interaction: mass spectrometry-based approaches. Curr. Opin. Biotechnol. 22, 42–49 - PubMed
- Fields S., Song O. (1989) A novel genetic system to detect protein–protein interactions. Nature 340, 245–246 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases