DYRK1A-mediated phosphorylation of GluN2A at Ser(1048) regulates the surface expression and channel activity of GluN1/GluN2A receptors - PubMed (original) (raw)
DYRK1A-mediated phosphorylation of GluN2A at Ser(1048) regulates the surface expression and channel activity of GluN1/GluN2A receptors
Cristina Grau et al. Front Cell Neurosci. 2014.
Abstract
N-methyl-D-aspartate glutamate receptors (NMDARs) play a pivotal role in neural development and synaptic plasticity, as well as in neurological disease. Since NMDARs exert their function at the cell surface, their density in the plasma membrane is finely tuned by a plethora of molecules that regulate their production, trafficking, docking and internalization in response to external stimuli. In addition to transcriptional regulation, the density of NMDARs is also influenced by post-translational mechanisms like phosphorylation, a modification that also affects their biophysical properties. We previously described the increased surface expression of GluN1/GluN2A receptors in transgenic mice overexpressing the Dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A), suggesting that DYRK1A regulates NMDARs. Here we have further investigated whether the density and activity of NMDARs were modulated by DYRK1A phosphorylation. Accordingly, we show that endogenous DYRK1A is recruited to GluN2A-containing NMDARs in the adult mouse brain, and we identify a DYRK1A phosphorylation site at Ser(1048) of GluN2A, within its intracellular C-terminal domain. Mechanistically, the DYRK1A-dependent phosphorylation of GluN2A at Ser(1048) hinders the internalization of GluN1/GluN2A, causing an increase of surface GluN1/GluN2A in heterologous systems, as well as in primary cortical neurons. Furthermore, GluN2A phosphorylation at Ser(1048) increases the current density and potentiates the gating of GluN1/GluN2A receptors. We conclude that DYRK1A is a direct regulator of NMDA receptors and we propose a novel mechanism for the control of NMDAR activity in neurons.
Keywords: DYRK1A; Down syndrome; GluN2A; NMDA receptor; phosphorylation; trafficking.
Figures
Figure 1
Dual specificity tyrosine-phosphorylation-regulated kinase 1A interacts with GluN2A and phosphorylates the GluN2A subunit at S1048. (A) Solubilized proteins from the adult mouse brain (input lane; 10% of lysates) were immunoprecipitated with either a mouse IgG or an anti-DYRK1A antibody, and both the lysates and the immunoprecipitates were analyzed in Western blots probed with anti-GluN2A and anti-DYRK1A antibodies as indicated. (B) Equivalent aliquots of anti-GluN1 purified immunocomplexes obtained from the adult mouse brain were analyzed in Western blots probed with an anti-GluN2A antibody (left panel) or they were used as the substrate in a radioactive in vitro phosphorylation assay in the presence or absence of purified recombinant GST-DYRK1A. The radiolabeled proteins were then fractionated by SDS-PAGE and detected by autoradiography (right panel). The arrows indicate the phosphorylated GluN2A and possibly, the phosphorylated GluN1, and the stars indicate the labeled bands resulting from GST-DYRK1A autophosphorylation. (C) Schematic representation of the GluN2A subunit topology and the GST fusion proteins covering the cytoplasmic tail of GluN2A used in the assays. (D) As indicated, bacterially purified GluN2A GST-fusions or unfused GST were examined in an IVK assay, in the presence of a GST fusion protein of the wild-type (wt) or kinase-deficient DYRK1A (KD). The substrates were analyzed by Coomassie staining (left panel) and the phosphorylated bands indicated by black arrows represent the full-length recombinant proteins, while the white arrows refer to the GluN2ACter truncated products and the asterisks indicate the GST-DYRK1Awt autophosphorylated bands (full-length or truncated products). (E) Schematic representation of the different mutant variants of the GST-GluN2AC1 fragment in which the asterisks indicate the position of the corresponding Ser to Ala mutants. (F) The GST-GluN2AC1 fragment or the indicated mutants were used as substrates in IVK assays with GST-DYRK1A. The panel shows a representative experiment and the histogram corresponds to the average 32P incorporation ± SEM (n = 2–3) calculated by densitometry (*p < 0.05). (G) The amino acid sequences of GluN2A from human (NP_000824), mouse (NP_032196), rat (NP_036705), dog (XP_005621613), sheep (XP_004020812) and chicken (XP_425252) were aligned to show the conserved region surrounding S1048 (marked with an asterisk). The numbers indicate the first and last amino acids listed.
Figure 2
Dual specificity tyrosine-phosphorylation-regulated kinase 1A mediated phosphorylation of GluN2A at S1048 increases the surface expression of GluN2A. COS-7 cells were transiently co-transfected with plasmids to express GluN1 and wild-type (wt), or mutant versions of GFP-GluN2A (S1048A, phospho-deficient; S1048E, phospho-mimetic), in the presence or absence of HA-DYRK1A (wt, wild-type; KD, kinase-inactive). After fixing, the cells were incubated with an anti-GFP antibody to label the surface receptors (blue). Direct green fluorescence was used to measure total GFP-GluN2A expression (green). HA-DYRK1A expressing cells were identified by anti HA-immunostaining (red). Scale bar = 10 µm. The histogram represents the mean ± SEM of the GluN2A surface expression normalized to the total GFP-GluN2A signal, with the values for transfections GluN1+GFP-GluN2Awt considered as 100 (n = 38–130 cells from, at least, three independent experiments per condition; *p < 0.05, ***p < 0.001, ANOVA).
Figure 3
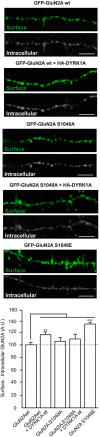
Dual specificity tyrosine-phosphorylation-regulated kinase 1A mediated phosphorylation of GluN2A at S1048 increases its surface expression in primary cortical neurons. Primary cultures of mouse embryo cortices were transiently transfected with GFP-GluN2A (wt, wild-type; S1048A, phospho-deficient mutant; S1048E, phospho-mimetic mutant) on day in vitro 8 (DIV8), in the presence or absence of heterologous HA-DYRK1A. The effect of DYRK1A on the surface:intracellular ratio of GluN1/GluN2A in primary mouse cortical neurons was evaluated by immunofluorescence. Prior to permeabilization, anti-GFP/Alexa488 was used to detect the surface chimeric receptors (represented in green), whereas intracellular GFP-GluN2A receptors were visualized after permeabilizing the cells, using an anti-GFP/Alexa555 antibody. Scale bar = 5 µm. The histogram represents the mean ± SEM GluN2A surface expression normalized to the intracellular GFP-GluN2A signal (n = 31–52 dendrites from, at least, three independent experiments per condition, *p < 0.05, **p < 0.01, ***p < 0.001, ANOVA).
Figure 4
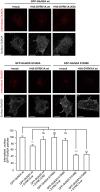
Reduction in the internalization rate of GluN2A in the presence of DYRK1A. Live transiently co-transfected COS-7 cells were incubated with an anti-GFP antibody for 30 min at 4°C. After the rapid removal of the excess antibody, the cells were placed in the incubator for additional 30 min at 37°C to allow the GluN2A-labeled particles to be internalized. Membrane receptors were then immunolabeled with Alexa647 (shown in gray), whereas the internalized receptors were immunolabeled with Alexa555 (red particles). Scale bar = 10 µm. Bottom, Histogram representing the mean ± SEM of the normalized ratio of the internalized particles and the surface GluN2A receptors (n = 31–115 cells from three independent experiments, ***p < 0.001, ANOVA).
Figure 5
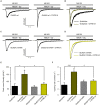
Dual specificity tyrosine-phosphorylation-regulated kinase 1A increases the NMDA-elicited current amplitude and opening rate. (A–D) Representative NMDA-elicited currents recorded from HEK-293T cells expressing HA-GluN1/HA-GluN2A receptors (panels A,B, wild-type GluN2A; panels C,D, GluN2AS1048A phospho-deficient mutant) in the presence (right) or absence (left) of GFP-DYRK1A. Whole-cell currents were elicited by perfusion of 1 mM NMDA with 50 µM glycine, in the continued presence of the open-channel blocker MK-801 (5 µM). (B,D) NMDA-evoked currents shown in panels (A) and (C) normalized to the same peak amplitude. (E) Average peak NMDA-evoked current density in cells transfected with NMDARs (GluN2Awt or GluN2AS1048A) in the presence or absence of DYRK1A (*p < 0.05, ANOVA followed by Bonferroni post hoc test). (F) Average opening rate of heterologously expressed NMDARs (GluN2Awt or GluN2AS1048A) in the presence or absence of DYRK1A (***p < 0.001 and *p < 0.05, ANOVA followed by a Bonferroni post hoc test).
Similar articles
- Protein Kinase C-Mediated Phosphorylation and α2δ-1 Interdependently Regulate NMDA Receptor Trafficking and Activity.
Zhou MH, Chen SR, Wang L, Huang Y, Deng M, Zhang J, Zhang J, Chen H, Yan J, Pan HL. Zhou MH, et al. J Neurosci. 2021 Jul 28;41(30):6415-6429. doi: 10.1523/JNEUROSCI.0757-21.2021. Epub 2021 Jun 17. J Neurosci. 2021. PMID: 34252035 Free PMC article. - Early expression of GluN2A-containing NMDA receptors in a model of fragile X syndrome.
Banke TG, Traynelis SF, Barria A. Banke TG, et al. J Neurophysiol. 2024 Apr 1;131(4):768-777. doi: 10.1152/jn.00406.2023. Epub 2024 Feb 21. J Neurophysiol. 2024. PMID: 38380828 Free PMC article. - GluN2A Subunit-Containing NMDA Receptors Are the Preferential Neuronal Targets of Homocysteine.
Sibarov DA, Abushik PA, Giniatullin R, Antonov SM. Sibarov DA, et al. Front Cell Neurosci. 2016 Nov 1;10:246. doi: 10.3389/fncel.2016.00246. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27847466 Free PMC article. - Hippocampal NMDA receptors and the previous experience effect on memory.
Cercato MC, Colettis N, Snitcofsky M, Aguirre AI, Kornisiuk EE, Baez MV, Jerusalinsky DA. Cercato MC, et al. J Physiol Paris. 2014 Sep-Dec;108(4-6):263-9. doi: 10.1016/j.jphysparis.2014.08.001. Epub 2014 Aug 15. J Physiol Paris. 2014. PMID: 25132342 Review. - Molecular bases of NMDA receptor subtype-dependent properties.
Glasgow NG, Siegler Retchless B, Johnson JW. Glasgow NG, et al. J Physiol. 2015 Jan 1;593(1):83-95. doi: 10.1113/jphysiol.2014.273763. Epub 2014 Sep 9. J Physiol. 2015. PMID: 25556790 Free PMC article. Review.
Cited by
- Dissecting the contribution of human chromosome 21 syntenic regions to recognition memory processes in adult and aged mouse models of Down syndrome.
Canonica T, Kidd EJ, Gibbins D, Lana-Elola E, Fisher EMC, Tybulewicz VLJ, Good M. Canonica T, et al. Front Behav Neurosci. 2024 Jul 10;18:1428146. doi: 10.3389/fnbeh.2024.1428146. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39050700 Free PMC article. - Impaired macroglial development and axonal conductivity contributes to the neuropathology of DYRK1A-related intellectual disability syndrome.
Pijuan I, Balducci E, Soto-Sánchez C, Fernández E, Barallobre MJ, Arbonés ML. Pijuan I, et al. Sci Rep. 2022 Nov 19;12(1):19912. doi: 10.1038/s41598-022-24284-5. Sci Rep. 2022. PMID: 36402907 Free PMC article. - Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications.
Lussier MP, Sanz-Clemente A, Roche KW. Lussier MP, et al. J Biol Chem. 2015 Nov 27;290(48):28596-603. doi: 10.1074/jbc.R115.652750. Epub 2015 Oct 9. J Biol Chem. 2015. PMID: 26453298 Free PMC article. Review. - Intellectual disability: dendritic anomalies and emerging genetic perspectives.
Quach TT, Stratton HJ, Khanna R, Kolattukudy PE, Honnorat J, Meyer K, Duchemin AM. Quach TT, et al. Acta Neuropathol. 2021 Feb;141(2):139-158. doi: 10.1007/s00401-020-02244-5. Epub 2020 Nov 23. Acta Neuropathol. 2021. PMID: 33226471 Free PMC article. Review. - Developing Medications Targeting Glutamatergic Dysfunction in Autism: Progress to Date.
Fung LK, Hardan AY. Fung LK, et al. CNS Drugs. 2015 Jun;29(6):453-63. doi: 10.1007/s40263-015-0252-0. CNS Drugs. 2015. PMID: 26104862 Free PMC article. Review.
References
- Altafaj X., Dierssen M., Baamonde C., Martí E., Visa J., Guimerà J., et al. . (2001). Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down’s syndrome. Hum. Mol. Genet. 10, 1915–1923. 10.1093/hmg/10.18.1915 - DOI - PubMed
- Altafaj X., Martín E. D., Ortiz-Abalia J., Valderrama A., Lao-Peregrín C., Dierssen M., et al. . (2013). Normalization of Dyrk1A expression by AAV2/1-shDyrk1A attenuates hippocampal-dependent defects in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 52, 117–127. 10.1016/j.nbd.2012.11.017 - DOI - PubMed
- Altafaj X., Ortiz-Abalia J., Fernández M., Potier M.-C., Laffaire J., Andreu N., et al. . (2008). Increased NR2A expression and prolonged decay of NMDA-induced calcium transient in cerebellum of TgDyrk1A mice, a mouse model of Down syndrome. Neurobiol. Dis. 32, 377–384. 10.1016/j.nbd.2008.07.024 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous