The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis - PubMed (original) (raw)
Review
The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis
Samuel S Duffy et al. Mult Scler Int. 2014.
Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterised by widespread areas of focal demyelination. Its aetiology and pathogenesis remain unclear despite substantial insights gained through studies of animal models, most notably experimental autoimmune encephalomyelitis (EAE). MS is widely believed to be immune-mediated and pathologically attributable to myelin-specific autoreactive CD4+ T cells. In recent years, MS research has expanded beyond its focus on CD4+ T cells to recognise the contributions of multiple immune and glial cell types to the development, progression, and amelioration of the disease. This review summarises evidence of T and B lymphocyte, natural killer cell, macrophage/microglial, astrocytic, and oligodendroglial involvement in both EAE and MS and the intercommunication and influence of each cell subset in the inflammatory process. Despite important advances in the understanding of the involvement of these cell types in MS, many questions still remain regarding the various subsets within each cell population and their exact contribution to different stages of the disease.
Figures
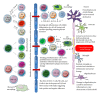
Figure 1
Immune and glial cell subtypes and their contributions to the pathogenesis of EAE and MS. During the development and progression of EAE and MS, a variety of cells representing both the innate and adaptive immune system breach the blood brain barrier and invade the brain parenchyma. Resident glial cells also become activated and play an important role in the pathogenesis of EAE and MS. Some of the cell types involved are proinflammatory and promote demyelination, axonal damage, and the formation of disease plaques, whilst other cell types have anti-inflammatory and/or regulatory properties and inhibit disease progression by facilitating tissue repair.
Similar articles
- Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE.
Van Kaer L, Postoak JL, Wang C, Yang G, Wu L. Van Kaer L, et al. Cell Mol Immunol. 2019 Jun;16(6):531-539. doi: 10.1038/s41423-019-0221-5. Epub 2019 Mar 15. Cell Mol Immunol. 2019. PMID: 30874627 Free PMC article. Review. - Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.
Rostami A, Ciric B. Rostami A, et al. J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review. - Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies.
Stinissen P, Raus J, Zhang J. Stinissen P, et al. Crit Rev Immunol. 1997;17(1):33-75. doi: 10.1615/critrevimmunol.v17.i1.20. Crit Rev Immunol. 1997. PMID: 9034723 Review. - Opioid growth factor and low-dose naltrexone impair central nervous system infiltration by CD4 + T lymphocytes in established experimental autoimmune encephalomyelitis, a model of multiple sclerosis.
Hammer LA, Waldner H, Zagon IS, McLaughlin PJ. Hammer LA, et al. Exp Biol Med (Maywood). 2016 Jan;241(1):71-8. doi: 10.1177/1535370215596384. Epub 2015 Jul 22. Exp Biol Med (Maywood). 2016. PMID: 26202376 Free PMC article. - Lack of junctional adhesion molecule (JAM)-B ameliorates experimental autoimmune encephalomyelitis.
Tietz S, Périnat T, Greene G, Enzmann G, Deutsch U, Adams R, Imhof B, Aurrand-Lions M, Engelhardt B. Tietz S, et al. Brain Behav Immun. 2018 Oct;73:3-20. doi: 10.1016/j.bbi.2018.06.014. Epub 2018 Jun 18. Brain Behav Immun. 2018. PMID: 29920328
Cited by
- Whole Exome Sequencing in Multi-Incident Families Identifies Novel Candidate Genes for Multiple Sclerosis.
Horjus J, van Mourik-Banda T, Heerings MAP, Hakobjan M, De Witte W, Heersema DJ, Jansen AJ, Strijbis EMM, de Jong BA, Slettenaar AEJ, Zeinstra EMPE, Hoogervorst ELJ, Franke B, Kruijer W, Jongen PJ, Visser LJ, Poelmans G. Horjus J, et al. Int J Mol Sci. 2022 Sep 28;23(19):11461. doi: 10.3390/ijms231911461. Int J Mol Sci. 2022. PMID: 36232761 Free PMC article. - Disrupted long-range gene regulations elucidate shared tissue-specific mechanisms of neuropsychiatric disorders.
Chen J, Song L, Yang A, Dong G, Zhao XM. Chen J, et al. Mol Psychiatry. 2022 Jun;27(6):2720-2730. doi: 10.1038/s41380-022-01529-3. Epub 2022 Apr 4. Mol Psychiatry. 2022. PMID: 35379909 - Therapeutic Potential of Ponesimod Alone and in Combination with Dimethyl Fumarate in Experimental Models of Multiple Sclerosis.
Pouzol L, Piali L, Bernard CC, Martinic MM, Steiner B, Clozel M. Pouzol L, et al. Innov Clin Neurosci. 2019 Mar 1;16(3-4):22-30. Innov Clin Neurosci. 2019. PMID: 31214480 Free PMC article. - A Peptide Targeting Inflammatory CNS Lesions in the EAE Rat Model of Multiple Sclerosis.
Boiziau C, Nikolski M, Mordelet E, Aussudre J, Vargas-Sanchez K, Petry KG. Boiziau C, et al. Inflammation. 2018 Jun;41(3):932-947. doi: 10.1007/s10753-018-0748-0. Inflammation. 2018. PMID: 29516383 - Culture of neural cells of the eyestalk of a mangrove crab is optimized on poly-L-ornithine substrate.
Wajsenzon IJ, de Carvalho LA, Biancalana A, da Silva WA, Dos Santos Mermelstein C, de Araujo EG, Allodi S. Wajsenzon IJ, et al. Cytotechnology. 2016 Oct;68(5):2193-206. doi: 10.1007/s10616-015-9942-1. Epub 2016 Jan 16. Cytotechnology. 2016. PMID: 26779908 Free PMC article.
References
- Browning V, Joseph M, Sedrak M. Multiple sclerosis: a comprehensive review for the physician assistant. Journal of the American Academy of Physician Assistants. 2012;25(8):24–29. - PubMed
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. The New England Journal of Medicine. 2000;343(13):938–952. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials