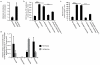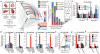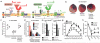Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS - PubMed (original) (raw)
Comparative Study
. 2014 Nov 20;515(7527):431-435.
doi: 10.1038/nature13909. Epub 2014 Nov 5.
Victoria R Pell # 2, Edoardo Gaude 3, Dunja Aksentijević 4, Stephanie Y Sundier 5, Ellen L Robb 1, Angela Logan 1, Sergiy M Nadtochiy 6, Emily N J Ord 7, Anthony C Smith 1, Filmon Eyassu 1, Rachel Shirley 7, Chou-Hui Hu 2, Anna J Dare 1, Andrew M James 1, Sebastian Rogatti 1, Richard C Hartley 8, Simon Eaton 9, Ana S H Costa 3, Paul S Brookes 6, Sean M Davidson 10, Michael R Duchen 5, Kourosh Saeb-Parsy 11, Michael J Shattock 4, Alan J Robinson 1, Lorraine M Work 7, Christian Frezza 3, Thomas Krieg 2, Michael P Murphy 1
Affiliations
- PMID: 25383517
- PMCID: PMC4255242
- DOI: 10.1038/nature13909
Comparative Study
Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS
Edward T Chouchani et al. Nature. 2014.
Abstract
Ischaemia-reperfusion injury occurs when the blood supply to an organ is disrupted and then restored, and underlies many disorders, notably heart attack and stroke. While reperfusion of ischaemic tissue is essential for survival, it also initiates oxidative damage, cell death and aberrant immune responses through the generation of mitochondrial reactive oxygen species (ROS). Although mitochondrial ROS production in ischaemia reperfusion is established, it has generally been considered a nonspecific response to reperfusion. Here we develop a comparative in vivo metabolomic analysis, and unexpectedly identify widely conserved metabolic pathways responsible for mitochondrial ROS production during ischaemia reperfusion. We show that selective accumulation of the citric acid cycle intermediate succinate is a universal metabolic signature of ischaemia in a range of tissues and is responsible for mitochondrial ROS production during reperfusion. Ischaemic succinate accumulation arises from reversal of succinate dehydrogenase, which in turn is driven by fumarate overflow from purine nucleotide breakdown and partial reversal of the malate/aspartate shuttle. After reperfusion, the accumulated succinate is rapidly re-oxidized by succinate dehydrogenase, driving extensive ROS generation by reverse electron transport at mitochondrial complex I. Decreasing ischaemic succinate accumulation by pharmacological inhibition is sufficient to ameliorate in vivo ischaemia-reperfusion injury in murine models of heart attack and stroke. Thus, we have identified a conserved metabolic response of tissues to ischaemia and reperfusion that unifies many hitherto unconnected aspects of ischaemia-reperfusion injury. Furthermore, these findings reveal a new pathway for metabolic control of ROS production in vivo, while demonstrating that inhibition of ischaemic succinate accumulation and its oxidation after subsequent reperfusion is a potential therapeutic target to decrease ischaemia-reperfusion injury in a range of pathologies.
Figures
Extended Data Figure 1
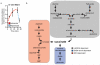
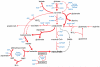
Comparative analysis of metabolites significantly accumulated in ischaemic conditions. a, Various murine tissues exposed to sufficient periods of ischaemia to prime for reperfusion ROS production were subjected to targeted LC-MS metabolomics analysis and comparison of metabolites that accumulated significantly when compared to normoxic levels. Following this, metabolites were scored according to the prevalence of their accumulation across five ischaemic tissue conditions. B = brain, H = whole heart ischaemia ex vivo, HL = LAD ischaemia in vivo, K = kidney, L = liver. b, Determination of linearity of the relationship between LC-MS metabolite peak intensity and concentration for CAC and related metabolites. c, Quality control determination of coefficient of variation for LC-MS quantification of CAC and related metabolites.
Extended Data Figure 2
Timecourse of succinate levels in the in vivo heart during ischaemia and reperfusion and potential metabolic inputs for succinate. a, Time course of succinate levels during myocardial ischaemia and reperfusion for the in vivo heart (5 min and 15 min ischaemia n = 4; 30 min ischaemia n = 9; 5 min reperfused n = 5). b, Summary of the three potential metabolic inputs for succinate-directed ischaemic flux. To understand the metabolic pathways that could contribute to succinate production under ischaemia, an updated version of the iAS253 model of cardiac metabolism was employed to simulate ischaemia using flux balance analysis. The model showed three possible mechanisms for producing succinate: from α-ketoglutarate produced by the CAC, derived from glycolysis, fatty acid oxidation, and glutaminolysis (grey box), from succinic semialdehyde produced from the GABA shunt (blue box), and from fumarate produced from the malate-aspartate shuttle and purine nucleotide cycle (red box) via the reversal of SDH. Data are shown as the mean ± s.e.m of at least four biological replicates.
Extended Data Figure 3
Metabolic labelling of CAC and proximal metabolites by 13C glucose in the ischaemic and normoxic myocardium. Proportional isotopic labelling profile of CAC and proximal metabolites during normoxic and ischaemic myocardial perfusion. Mouse hearts were perfused with 11 mM 13C glucose for 10 min followed by either 30 min no flow ischaemia or 30 min normoxic perfusion followed by snap-freezing and LC-MS metabolomic analysis (n = 4). * p < 0.05, ** p < 0.01, *** p < 0.001. Data are shown as the mean ± s.e.m of at least four biological replicates.
Extended Data Figure 4
Metabolic labelling of CAC and proximal metabolites by 13C palmitate in the ischaemic and normoxic myocardium a, Mouse hearts were perfused with 0.3 mM 13C palmitate (+16 labelled) for 10 min resulting in a significant proportion of the endogenous palmitate pool being +16 labelled. Following this, hearts were subjected to either 30 min ischaemia or continued normoxic respiration with 13C palmitate followed by snap-freezing and metabolomic analysis. b, Isotopic flux from palmitate to CAC and proximal metabolites following normoxic and ischaemic myocardial respiration. The isotopic profile for each metabolite is expressed as a proportion of the total pool (n = 4). * p < 0.05, ** p < 0.01, *** p < 0.001. Data are shown as the mean ± s.e.m of at least four biological replicates.
Extended Data Figure 5
Metabolic labelling of CAC and proximal metabolites by 13C glutamine in the ischaemic and normoxic myocardium, and measurement of the effect of inhibition of GABA transaminase succinate accumulation in the ischaemic myocardium. a, Mouse hearts were perfused with 4 mM 13C glutamine (+5 labelled) for 10 min followed by either 30 min no flow ischaemia or 30 min normoxic respiration followed by snap freezing and metabolomic analysis. The isotopic profile for each metabolite is expressed as a proportion of the total pool (n=4) b, Additionally, flux to α-KG was determined relative to the proportion of the +5 glutamine pool in the heart (n = 4). c, Perfused mouse hearts were subjected to 30 min no flow ischaemia ± continuous infusion of vigabatrin (Vig; 100, 300, and 700 μM) 10 min prior to ischaemia. Heart tissue was snap frozen and c, GABA and d, succinate abundance quantified relative to normoxic levels by LC-MS (n = 4; ischaemia n = 5). * p < 0.05. Data are shown as the mean ± s.e.m of at least four biological replicates.
Extended Data Figure 6
Unabridged metabolic model identifying pathways that can become activated by tissue ischaemia to drive succinate accumulation. To identify the metabolic pathways that could contribute to succinate production under ischaemia, we simulated these conditions using flux balance analysis in conjunction with an expanded version of the iAS253 mitochondrial model of central cardiac metabolism. The major pathways contributing to succinate accumulation (bold red lines) were via fumarate feeding into the reverse activity of SDH. This was produced by the purine nucleotide cycle (PNC) and the malate-aspartate shuttle (MAS), which consumed glucose and aspartate, and also led to significant production of lactate and alanine. Lesser sources of succinate (thin red lines) included glycolysis and glutaminolysis but this was relatively minor as this route was constrained by the overproduction of NADH. In addition a small amount of fumarate was generated by pyruvate carboxylase activity. The GABA shunt did not contribute (black dashed line).
Extended Data Figure 7
Effects of dimethyl malonate and dimethyl succinate treatment of cells and in vivo on intracellular accumulation of malonate and succinate, and respiration and comparison of 13C ischaemic metabolite fluxes to succinate relative to isotopic donor pools. a, Intravenous infusion of dimethyl malonate in vivo results in accumulation of malonate in the ischaemic myocardium (n = 4). b,c, C2C12 cells were incubated with: no additions, glucose, 5 mM dimethyl succinate, 5 mM dimethyl malonate, or 5 mM dimethyl malonate and 5 mM dimethyl succinate. Cellular oxygen consumption rate due to (b) ATP synthesis and (c) maximal rates in the presence of FCCP were determined using a Seahorse XF96 analyser (n = 4). d, Mouse hearts were perfused with 13C-glucose (+6 labelled), 13C-glutamine (+5 labelled), 13C-aspartate (+1 labelled), or 13C-palmitate (+16 labelled) for 10 min followed by 30 min no flow ischaemia or 30 min normoxic respiration, followed by snap-freezing and metabolomic analysis. To compare the relative magnitude of metabolite flux from each carbon source, 13C incorporation to succinate during normoxia and ischaemia was determined relative to the proportion of the labelled pool of the relevant infused 13C donor. 13C incorporation into succinate was considered in terms of the proportion of the +4 isotope in the entire succinate pool for 13C-glucose, 13C-glutamine and 13C-palmitate infusions; and the proportion of the +1 isotope in the entire pool for the 13C-aspartate infusion. (n = 4), *, p < 0.05; ***, p < 0.001. Data are shown as the mean ± s.e.m of at least four biological replicates.
Extended Data Figure 8
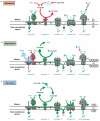
Predicted changes in pathways of succinate and OXPHOS metabolism during ischaemia and following reperfusion. To determine possible changes in succinate metabolism during ischaemia, reperfusion and normoxia, cardiac metabolism was simulated in these conditions using an expanded version of the iAS253 model with flux balance analysis. The simulations predicted that: a, under ischaemia, complex II ran in reverse by using ubiquinol produced by complex I to reduce fumarate to succinate, thereby acting as a terminal electron acceptor instead of oxygen. Fumarate was produced from the purine nucleotide cycle (PNC) and reversal of the citric acid cycle (CAC). Flux through the rest of the respiratory chain was diminished and AMP was produced from ADP due to insufficient ATP production. b, With oxygen restored complex II metabolised excess succinate. A delay in regenerating AMP to ADP, as typified in the first minute of reperfusion, limited the flux through ATP-synthase. This in turn prevented complex III consuming all the ubiquinol generated by complex II, as the membrane became hyper-polarised. The excess flux of ubiquinol and protons forced complex I to run in reverse, which would generate ROS by RET. c, Once the flux of succinate was reduced to normal levels, as in the transition from late reperfusion to normoxia, the fluxes through the respiratory chain and citric acid cycle returned to normal.
Extended Data Figure 9
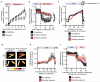
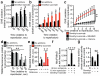
Tracking DHE oxidation, NAD(P)H reduction state, and mitochondrial membrane potential in primary cardiomyocytes during in situ IR. a, Inhibition of mitochondrial complex I RET reduces DHE oxidation on reperfusion (n = 6; rotenone n =4). b,c Effect of manipulation of ischaemic succinate levels on NAD(P)H oxidation during early reperfusion (n = 3). Primary rat cardiomyocytes were subjected to 40 min ischaemia followed by reoxygenation and NAD(P)H reduction state was tracked throughout the experiment by measurement of NAD(P)H autofluorescence. Ischaemic buffer contained either no additions, 4 mM dimethyl malonate, or 4 mM dimethyl succinate. Average (b) and representative (c) traces from each condition are shown. The highlighted window in (c) indicates the period of the experiment expanded in detail in (b). d, Effect of inhibition of ischaemic succinate accumulation on mitochondrial membrane potential following late ischaemia (left panels) and early reperfusion (right panels). e,f, Primary rat cardiomyocytes were subjected to 40 min ischaemia and reoxygenation and mitochondrial membrane potential was tracked throughout the experiment by measurement of tetramethylrhoadmine (TMRM) fluorescence. Ischaemic buffer contained either no additions or 4 mM dimethyl malonate. (e), TMRM signal throughout the entire experiment. (f) TMRM signal during the transition from ischaemia to reoxygenation (n = 3). Data are shown as the mean ± s.e.m of at least three biological replicates. Replicates represent separate experiments on independent cell preparations.
Extended Data Figure 10
Quantification of CAC intermediates in the heart following infusion of dimethyl succinate and in the brain following infusion of dimethyl malonate, and extended summary cytoprotection and neurological scores of rats subjected to tMCAO IR in vivo ± dimethyl malonate infusion. a, Effect of intravenous infusion of dimethyl succinate on CAC metabolite abundance in the ischaemic and non-ischaemic myocardium(normoxia and peripheral heart tissue + dimethyl succinate n = 3; ischaemia + dimethyl succinate n = 4; α-KG and aconitate in peripheral heart tissue n = 2). b, Profile of mitochondrial CAC metabolite levels following tMCAO ischaemia ± dimethyl malonate (n = 4). c, Representative images of cross-sections from rat brains after undergoing tMCAO in vivo ± treatment with dimethyl malonate. Brains were treated with hematoxylin and eosin to delineate infarcted tissue. d, Locomotor and sensorimotor assessment of rats by quantification of average number of footfalls following tCMAO ± dimethyl malonate (control n = 6; dimethyl malonate n = 4). *, p < 0.05; **, p < 0.01. Data are shown as the mean ± s.e.m of at least three biological replicates, unless otherwise stated.
Figure 1
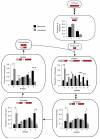
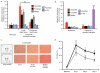
Comparative metabolomics identifies succinate as a potential mitochondrial metabolite that drives reperfusion ROS production. a, Comparative metabolomics strategy. b, HIVE plot comparative analysis. All identified metabolites are identified on the horizontal axis, while those accumulated (top axis) or depleted (bottom axis) in a particular ischaemic tissue are indicated by a connecting arc. Metabolites accumulated commonly across all tissues are highlighted. c, Prevalence of accumulation of metabolites in murine tissues during ischaemia. d, Profile of mitochondrial CAC metabolite levels following ischaemia across five ischaemic tissue conditions. (in vivo heart n = 5; succinate and fumarate n = 9), (ex vivo heart n = 4), (liver n = 4), (brain n = 3), (kidney n = 4). e, Time course of CAC metabolite levels during myocardial ischaemia and reperfusion for 5 min in the ex vivo heart (n = 4). f, CAC metabolite levels during in vivo myocardial IR in at risk and peripheral heart tissue following ischaemia and after 5 min reperfusion. (n = 5; succinate and fumarate n = 9). g, CAC metabolite levels during in vivo brain IR following ischaemia and following 5 min reperfusion (n = 3). h, CAC metabolite levels during in vivo kidney IR following ischaemia and after 5 min reperfusion (n = 4; aconitate n = 3). ** p < 0.01, *** p < 0.001. Data are shown as the mean ± s.e.m of at least three biological replicates.
Figure 2
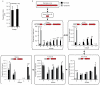
Reverse SDH activity drives ischaemic succinate accumulation by the reduction of fumarate. a, Potential inputs to succinate-directed flux by conventional cardiac metabolism and 13C-metabolite labelling strategy. b, c, 13C isotopologue profile of succinate in the normoxic and ischaemic myocardium following infusion of (b) 13C-glucose and (c) 13C-palmitate (n = 4). d, Effect of inhibition of GABA shunt with vigabatrin on GABA and succinate levels in the ischaemic myocardium (n = 4; ischaemia n = 5). e, Summary of in silico metabolic modelling of potential drivers of ischaemic succinate accumulation, and 13C-aspartate metabolic labelling strategy. f, Effect of SDH inhibition by dimethyl malonate on CAC metabolite abundance in the ischaemic myocardium in vivo (n = 3). g, Relative incorporation of 13C-aspartate to the indicated CAC metabolites in the normoxic and ischaemic myocardium (n = 4). h, Effect on CAC metabolite abundance in the ischaemic myocardium in vivo of blocking aspartate entry into the CAC through AOA-mediated inhibition of aspartate aminotransferase, or blocking PNC by inhibition of adenylosuccinate lyase with AICAR (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001. Data are shown as the mean ± s.e.m of at least three biological replicates.
Figure 3
Ischaemic succinate levels control ROS production in adult primary cardiomyocytes and in the heart in vivo. a, b, DHE oxidation during late ischaemia and early reperfusion ± (a) inhibition of ischaemic succinate accumulation (no additions n = 6; dimethyl malonate n = 5) or (b) addition of dimethyl succinate during ischaemia (n = 6). c, Inhibition of mitochondrial complex I RET reduces DHE oxidation on reperfusion following addition of dimethyl succinate. Ischaemia (Isch). (n = 5; dimethyl succinate n = 6). d, Effect of dimethyl malonate on mitochondrial repolarisation at reperfusion as determined by the rate of TMRM quenching (n = 3). e, Effect of dimethyl succinate and oligomycin on mitochondrial ROS in aerobic C2C12 myoblasts (n = 4). f, g. Effect of inhibition of ischaemic succinate accumulation by dimethyl malonate on mitochondrial ROS during IR injury in vivo assessed by (f) MitoB oxidation (n = 5; dimethyl malonate n = 6), and by (g) aconitase inactivation (n = 4). * p < 0.05, ** p < 0.01. Data are shown as the mean ± s.e.m of at least three biological replicates. For cell data replicates represent separate experiments on independent cell preparations.
Figure 4
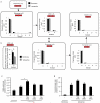
NADH and AMP sensing pathways drive ischaemic succinate accumulation to control reperfusion pathologies in vivo through mitochondrial ROS production. a, Model of succinate accumulation during ischaemia and superoxide formation by RET during reperfusion. b, Representative cross-sections from mouse hearts following myocardial infarction ± inhibition of ischaemic succinate accumulation and reintroduction of ischaemic succinate. Infarcted tissue is white, the rest of the area at risk is red, and non-risk tissue is dark blue. c, Quantification of myocardial infarct size as described in (b). (n = 6). d, Effect of i.v infusion of dimethyl succinate in combination with SDH inhibition by dimethyl malonate on CAC metabolite abundance in the ischaemic myocardium in vivo (n = 4). e, Effect of i.v infusion of dimethyl malonate on succinate accumulation in the ischaemic brain in vivo (n = 4). f-h, Protection by dimethyl malonate against brain IR injury in vivo. Quantification of brain infarct volume (f) and rostro-caudal infarct distribution (g) ± dimethyl malonate following brain IR injury by tMCAO in vivo (untreated n = 6; dimethyl malonate n = 4). (h) Neurological scores for rats following tMCAO ± dimethyl malonate (untreated n = 6; dimethyl malonate n = 4). * p < 0.05, ** p < 0.01, *** p < 0.001. Data are shown as the mean ± s.e.m of at least three biological replicates, except for h, for which data are median ± C.I.
Comment in
- Biochemistry: succinate strikes.
O'Neill LA. O'Neill LA. Nature. 2014 Nov 20;515(7527):350-1. doi: 10.1038/nature13941. Epub 2014 Nov 5. Nature. 2014. PMID: 25383526 No abstract available. - Mitochondrial metabolomics unravel the primordial trigger of ischemia/reperfusion injury.
Heger M, Reiniers MJ, van Golen RF. Heger M, et al. Gastroenterology. 2015 May;148(5):1071-3. doi: 10.1053/j.gastro.2015.03.041. Epub 2015 Mar 28. Gastroenterology. 2015. PMID: 25824356 No abstract available. - Succinate: A Promising Therapeutic Target for Reperfusion Injury.
Ozpinar A, Weiner GM, Ducruet AF. Ozpinar A, et al. Neurosurgery. 2015 Dec;77(6):N13-4. doi: 10.1227/01.neu.0000473807.30361.29. Neurosurgery. 2015. PMID: 26584319 No abstract available. - Uncovering the origin of oxidative damage in ischaemia-reperfusion injury in the heart.
Figueroa-Juárez E. Figueroa-Juárez E. Nat Rev Endocrinol. 2023 Oct;19(10):560. doi: 10.1038/s41574-023-00881-w. Nat Rev Endocrinol. 2023. PMID: 37495726 No abstract available.
Similar articles
- A model of mitochondrial superoxide production during ischaemia-reperfusion injury for therapeutic development and mechanistic understanding.
Sorby-Adams A, Prime TA, Miljkovic JL, Prag HA, Krieg T, Murphy MP. Sorby-Adams A, et al. Redox Biol. 2024 Jun;72:103161. doi: 10.1016/j.redox.2024.103161. Epub 2024 Apr 24. Redox Biol. 2024. PMID: 38677214 Free PMC article. - Pre-ischaemic mitochondrial substrate constraint by inhibition of malate-aspartate shuttle preserves mitochondrial function after ischaemia-reperfusion.
Jespersen NR, Yokota T, Støttrup NB, Bergdahl A, Paelestik KB, Povlsen JA, Dela F, Bøtker HE. Jespersen NR, et al. J Physiol. 2017 Jun 15;595(12):3765-3780. doi: 10.1113/JP273408. Epub 2017 Feb 27. J Physiol. 2017. PMID: 28093764 Free PMC article. - Increased Succinate Accumulation Induces ROS Generation in In Vivo Ischemia/Reperfusion-Affected Rat Kidney Mitochondria.
Kamarauskaite J, Baniene R, Trumbeckas D, Strazdauskas A, Trumbeckaite S. Kamarauskaite J, et al. Biomed Res Int. 2020 Oct 13;2020:8855585. doi: 10.1155/2020/8855585. eCollection 2020. Biomed Res Int. 2020. PMID: 33102598 Free PMC article. - Succinate metabolism: a new therapeutic target for myocardial reperfusion injury.
Pell VR, Chouchani ET, Frezza C, Murphy MP, Krieg T. Pell VR, et al. Cardiovasc Res. 2016 Jul 15;111(2):134-41. doi: 10.1093/cvr/cvw100. Epub 2016 May 18. Cardiovasc Res. 2016. PMID: 27194563 Review. - The role of succinate and ROS in reperfusion injury - A critical appraisal.
Andrienko TN, Pasdois P, Pereira GC, Ovens MJ, Halestrap AP. Andrienko TN, et al. J Mol Cell Cardiol. 2017 Sep;110:1-14. doi: 10.1016/j.yjmcc.2017.06.016. Epub 2017 Jul 5. J Mol Cell Cardiol. 2017. PMID: 28689004 Free PMC article. Review.
Cited by
- Ischemia-induced endogenous Nrf2/HO-1 axis activation modulates microglial polarization and restrains ischemic brain injury.
Kuo PC, Weng WT, Scofield BA, Paraiso HC, Yu II, Yen JJ. Kuo PC, et al. Front Immunol. 2024 Oct 14;15:1440592. doi: 10.3389/fimmu.2024.1440592. eCollection 2024. Front Immunol. 2024. PMID: 39469715 Free PMC article. - Targeting ER stress and calpain activation to reverse age-dependent mitochondrial damage in the heart.
Thompson J, Maceyka M, Chen Q. Thompson J, et al. Mech Ageing Dev. 2020 Dec;192:111380. doi: 10.1016/j.mad.2020.111380. Epub 2020 Oct 9. Mech Ageing Dev. 2020. PMID: 33045249 Free PMC article. Review. - UCP2, a Member of the Mitochondrial Uncoupling Proteins: An Overview from Physiological to Pathological Roles.
Nesci S, Rubattu S. Nesci S, et al. Biomedicines. 2024 Jun 13;12(6):1307. doi: 10.3390/biomedicines12061307. Biomedicines. 2024. PMID: 38927514 Free PMC article. Review. - Cardioprotective Effects of Mitochondria-Targeted Peptide SBT-20 in two Different Models of Rat Ischemia/Reperfusion.
Dai W, Cheung E, Alleman RJ, Perry JB, Allen ME, Brown DA, Kloner RA. Dai W, et al. Cardiovasc Drugs Ther. 2016 Dec;30(6):559-566. doi: 10.1007/s10557-016-6695-9. Cardiovasc Drugs Ther. 2016. PMID: 27747447 Free PMC article. - Decreased ability to manage increases in reactive oxygen species may underlie susceptibility to arrhythmias in mice lacking Scn1b.
Aldridge JL, Alexander ED, Franklin AA, Frasier CR. Aldridge JL, et al. Am J Physiol Heart Circ Physiol. 2024 Oct 1;327(4):H723-H732. doi: 10.1152/ajpheart.00265.2024. Epub 2024 Aug 9. Am J Physiol Heart Circ Physiol. 2024. PMID: 39120465
References
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. - PubMed
- Timmers L, et al. The innate immune response in reperfused myocardium. Cardiovasc. Res. 2012;94:276–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PG/12/42/29655/BHF_/British Heart Foundation/United Kingdom
- MC_U105674181/MRC_/Medical Research Council/United Kingdom
- R01 HL071158/HL/NHLBI NIH HHS/United States
- MC_UU_12022/6/MRC_/Medical Research Council/United Kingdom
- RG/12/4/29426/BHF_/British Heart Foundation/United Kingdom
- MC_U105663142/MRC_/Medical Research Council/United Kingdom
- CAPMC/ CIHR/Canada
- PG/07/126/24223/BHF_/British Heart Foundation/United Kingdom
- G1100562/MRC_/Medical Research Council/United Kingdom
- MC_UP_1101/3/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources