Putative chanzyme activity of TRPM2 cation channel is unrelated to pore gating - PubMed (original) (raw)
Putative chanzyme activity of TRPM2 cation channel is unrelated to pore gating
Balázs Tóth et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Transient receptor potential melastatin 2 (TRPM2) is a Ca(2+)-permeable cation channel expressed in immune cells of phagocytic lineage, pancreatic β cells, and brain neurons and is activated under oxidative stress. TRPM2 activity is required for immune cell activation and insulin secretion and is responsible for postischemic neuronal cell death. TRPM2 is opened by binding of ADP ribose (ADPR) to its C-terminal cytosolic nudix-type motif 9 (NUDT9)-homology (NUDT9-H) domain, which, when expressed in isolation, cleaves ADPR into AMP and ribose-5-phosphate. A suggested coupling of this enzymatic activity to channel gating implied a potentially irreversible gating cycle, which is a unique feature of a small group of channel enzymes known to date. The significance of such a coupling lies in the conceptually distinct pharmacologic strategies for modulating the open probability of channels obeying equilibrium versus nonequilibrium gating mechanisms. Here we examine the potential coupling of TRPM2 enzymatic activity to pore gating. Mutation of several residues proposed to enhance or eliminate NUDT9-H catalytic activity all failed to affect channel gating kinetics. An ADPR analog, α-β-methylene-ADPR (AMPCPR), was shown to be entirely resistant to hydrolysis by NUDT9, but nevertheless supported TRPM2 channel gating, albeit with reduced apparent affinity. The rate of channel deactivation was not slowed but, rather, accelerated in AMPCPR. These findings, as well as detailed analyses of steady-state gating kinetics of single channels recorded in the presence of a range of concentrations of ADPR or AMPCPR, identify TRPM2 as a simple ligand-gated channel that obeys an equilibrium gating mechanism uncoupled from its enzymatic activity.
Keywords: ADP ribose analog; Nudix motif; channel enzyme; ligand-gated channel; nonequilibrium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Mutations of residues proposed to be key for catalysis have little effect on TRPM2 channel gating. (A, C, D_–_F, H_–_J) Inward macroscopic currents elicited by application of 125 μM Ca2+ (black bars) plus 1 or 32 μM ADPR (gray staggered bars) to the cytosolic faces of inside-out patches excised from Xenopus oocytes expressing human (A) WT, (C) D1468A, (D) Q1408K, (E) E1409K, (F) Q1408K/E1409K, (H) I1405E, (I) L1406F, and (J) I1405E/L1406F TRPM2 channels. Colored lines, single exponential fits to current relaxation time courses; τ, time constants. (B) Sequence alignment of NUDT9 and NUDT9-H Nudix boxes highlighting residues functionally important in NUDT9. (G) Fractional current activation (mean ± SEM) by 1 μM ADPR for WT and mutant TRPM2; steady current in 1 μM ADPR was normalized to that in 32 μM ADPR in the same patch. (K) Deactivation time constants (mean ± SEM) after ADPR removal, from single-exponential fits.
Fig. 2.
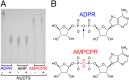
AMPCPR is resistant to hydrolysis. (A) TLC analysis of ADPR, AMP, and AMPCPR samples before (−) and after (+) incubation with purified NUDT9 (see SI Materials and Methods). ADPR is degraded into AMP and ribose-5-phosphate; the latter is not visible on the TLC. (B) Structures of ADPR and AMPCPR. Vertical arrow highlights the oxygen bridge in ADPR that is cleaved by NUDT9.
Fig. 3.
Nonhydrolyzable ADPR analog is a low-affinity partial TRPM2 channel agonist. (A and B) T5L-TRPM2 currents are stimulated in a dose-dependent manner by cytosolic application of either (A) 0.32, 1, 3.2, 10, and 32 μM ADPR (blue staggered bar) or (B) 10, 32, and 200 μM AMPCPR (red staggered bar) in the presence of saturating cytosolic Ca2+ (black bars). Currents elicited in the same patches by saturating (32 μM) ADPR (blue bars) were used for normalization. (C) Normalized dose–response curves (mean ± SEM) for stimulation of macroscopic T5L-TRPM2 current by ADPR (blue symbols; replotted from ref. 11) and AMPCPR (red symbols). Solid lines are fits to the Hill equation.
Fig. 4.
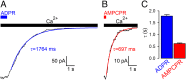
Nonhydrolyzable ligand does not slow TRPM2 channel deactivation. (A and B) Decay time courses on nucleotide removal of macroscopic T5L-TRPM2 currents activated by quasi-saturating concentrations of (A) ADPR or (B) AMPCPR in the presence of saturating Ca2+. Colored lines, single-exponential fits; τ, time constants. (C) Mean ± SEM deactivation time constants of T5L-TRPM2 channels opened by ADPR and AMPCPR.
Fig. 5.
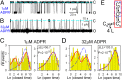
Bursting gating pattern of single T5L-TRPM2 channels is evident even in saturating ADPR. (A and B) Current recordings from single T5L-TRPM2 channels in 125 μM cytosolic Ca2+ and (A) 1 or (B) 32 μM ADPR. Dashed lines, zero-current level. (C and D) Histograms (22) of closed (Left) and open (Right) dwell times of single channels gating in (C) 1 or (D) 32 μM ADPR, from the recordings in A and B, respectively. Dashed blue and solid red lines are maximum-likelihood fits to the dwell-time distributions by subsets of the scheme in E, framed in respective colors. Log-likelihood ratios (ΔLL) allow rejection of the C↔O model with significance P (29); similar ΔLL values (31.5–310.1) were obtained in six further single-channel patches. (E) Simplest gating scheme compatible with the dwell-time distributions. Boxed subsets generate identically colored fits in C and D. Asterisks mark fully liganded states. Cs*, long (slow) closed state; O*, open state; Cf*, brief (flickery) closed state. Horizontal dashed double arrow connects unliganded (Cs) and fully liganded (Cs*) long closed states and represents sequential binding of four ligands.
Fig. 6.
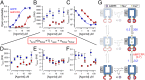
Detailed kinetic analysis reveals molecular mechanism of nucleotide-dependent channel activation. (A_–_F) Dose–response curves (mean ± SEM) of (A) _P_o, (B) τb, (C) τib, (D) τo, (E) τf, and (F) nf, for T5L-TRPM2 channels gating at steady state in 125 μM Ca2+ plus various concentrations of ADPR or AMPCPR (blue and red symbols, respectively), extracted by maximum-likelihood fitting of 199 well-resolved multichannel recordings from 124 patches (see Materials and Methods and Fig. S4). Note that τb is a function of the intraburst parameters in D_–_F (equation in red box). Solid lines in A are fits to the Hill equation [_P_o = _P_o;∞·([L]n/([L]n+Kn)]; those in C are fits to the inverse of the Hill equation [τib = ([L]n+Kn)/([L]n·τib;∞)]. Fit parameters were (A) _P_o;∞ = 0.75 ± 0.03, K = 2.1 ± 0.3 μM, n = 1.0 ± 0.1 for ADPR and _P_o;∞ = 0.41 ± 0.02, K = 48 ± 6 μM, n = 2.5 ± 0.5 for AMPCPR; (C) τib;∞ = 1.0 ± 0.2 s, K = 7.1 ± 1.9 μM, n = 1.5 ± 0.1 for ADPR and τib;∞ = 1.8 ± 0.8 s, K = 83 ± 45 μM, n = 1.4 ± 0.1 for AMPCPR. (G) Cartoon, molecular interpretation of nucleotide-dependent TRPM2 gating. Numbers on vertical transitions are rates (s−1). Blue, TM domain; upper constriction, selectivity filter; lower constriction, TM6 bundle crossing; red, NUDT9H domains; purple, activating nucleotides; yellow, Ca2+; green, Na+. Incomplete occlusion of the nucleotide might allow for ligand release in the bursting state (to putative states depicted in faint print).
Similar articles
- Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2.
Kühn FJ, Lückhoff A. Kühn FJ, et al. J Biol Chem. 2004 Nov 5;279(45):46431-7. doi: 10.1074/jbc.M407263200. Epub 2004 Sep 3. J Biol Chem. 2004. PMID: 15347676 - The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity.
Iordanov I, Mihályi C, Tóth B, Csanády L. Iordanov I, et al. Elife. 2016 Jul 6;5:e17600. doi: 10.7554/eLife.17600. Elife. 2016. PMID: 27383051 Free PMC article. - Structures and gating mechanism of human TRPM2.
Wang L, Fu TM, Zhou Y, Xia S, Greka A, Wu H. Wang L, et al. Science. 2018 Dec 21;362(6421):eaav4809. doi: 10.1126/science.aav4809. Epub 2018 Nov 22. Science. 2018. PMID: 30467180 Free PMC article. - TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose.
Kühn FJ, Heiner I, Lückhoff A. Kühn FJ, et al. Pflugers Arch. 2005 Oct;451(1):212-9. doi: 10.1007/s00424-005-1446-y. Epub 2005 Jun 11. Pflugers Arch. 2005. PMID: 15952035 Review. - TRPM2.
Eisfeld J, Lückhoff A. Eisfeld J, et al. Handb Exp Pharmacol. 2007;(179):237-52. doi: 10.1007/978-3-540-34891-7_14. Handb Exp Pharmacol. 2007. PMID: 17217061 Review.
Cited by
- Activators of TRPM2: Getting it right.
Rosenbaum T. Rosenbaum T. J Gen Physiol. 2015 Jun;145(6):485-7. doi: 10.1085/jgp.201511405. Epub 2015 May 11. J Gen Physiol. 2015. PMID: 25964430 Free PMC article. No abstract available. - Novel Alleles of gon-2, a C. elegans Ortholog of Mammalian TRPM6 and TRPM7, Obtained by Genetic Reversion Screens.
Lambie EJ, Bruce RD 3rd, Zielich J, Yuen SN. Lambie EJ, et al. PLoS One. 2015 Nov 25;10(11):e0143445. doi: 10.1371/journal.pone.0143445. eCollection 2015. PLoS One. 2015. PMID: 26606136 Free PMC article. - Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel.
Huang Y, Roth B, Lü W, Du J. Huang Y, et al. Elife. 2019 Sep 12;8:e50175. doi: 10.7554/eLife.50175. Elife. 2019. PMID: 31513012 Free PMC article. - TRPM2 ion channels steer neutrophils towards a source of hydrogen peroxide.
Morad H, Luqman S, Tan CH, Swann V, McNaughton PA. Morad H, et al. Sci Rep. 2021 Apr 29;11(1):9339. doi: 10.1038/s41598-021-88224-5. Sci Rep. 2021. PMID: 33927223 Free PMC article. - Dual amplification strategy turns TRPM2 channels into supersensitive central heat detectors.
Bartók Á, Csanády L. Bartók Á, et al. Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2212378119. doi: 10.1073/pnas.2212378119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36409885 Free PMC article.
References
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87(1):165–217. - PubMed
- McQuillin A, et al. Fine mapping of a susceptibility locus for bipolar and genetically related unipolar affective disorders, to a region containing the C21ORF29 and TRPM2 genes on chromosome 21q22.3. Mol Psychiatry. 2006;11(2):134–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous