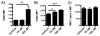The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? - PubMed (original) (raw)
The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant?
Sesquile Ramon et al. J Immunol. 2014.
Abstract
Influenza viruses remain a critical global health concern. More efficacious vaccines are needed to protect against influenza virus, yet few adjuvants are approved for routine use. Specialized proresolving mediators (SPMs) are powerful endogenous bioactive regulators of inflammation, with great clinical translational properties. In this study, we investigated the ability of the SPM 17-HDHA to enhance the adaptive immune response using an OVA immunization model and a preclinical influenza vaccination mouse model. Our findings revealed that mice immunized with OVA plus 17-HDHA or with H1N1-derived HA protein plus 17-HDHA increased Ag-specific Ab titers. 17-HDHA increased the number of Ab-secreting cells in vitro and the number of HA-specific Ab-secreting cells present in the bone marrow. Importantly, the 17-HDHA-mediated increased Ab production was more protective against live pH1N1 influenza infection in mice. To our knowledge, this is the first report on the biological effects of ω-3-derived SPMs on the humoral immune response. These findings illustrate a previously unknown biological link between proresolution signals and the adaptive immune system. Furthermore, this work has important implications for the understanding of B cell biology, as well as the development of new potential vaccine adjuvants.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
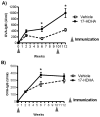
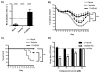
Figure 1. 17-HDHA increases OVA-specific antibody production
C57Bl/6 mice were immunized i.p. at week 0 and at week 10 with OVA plus vehicle control (vehicle) or with OVA plus 1 μg 17-HDHA (17-HDHA). Sera were collected at weeks 2, 6 and 12. OVA-specific A) IgM and B) IgG titers were measured by ELISA. Here is shown mean ±SEM (n=6/group). Statistical analysis done using two-way ANOVA (*p≤0.05).
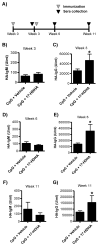
Figure 2. 17-HDHA increases HA-specific IgG production
C57Bl/6 mice were immunized i.m. with A/Brisbane/59/2007 recombinant HA plus CpG plus vehicle control (CpG + vehicle), or with A/Brisbane/59/2007 recombinant HA plus CpG and 1 μg 17-HDHA (CpG + 17-HDHA) and sera was collected as represented in A) immunization schematic. HA-specific IgM and HA-specific IgG antibody levels were measured by ELISA at B–C) week 3, D–E) week 6 and F–G) week 11 (n=6/group). Data shown as mean ±SEM. Statistical analysis done using unpaired Student’s t-test (*p≤0.05).
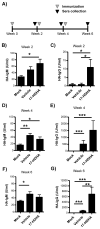
Figure 3. 17-HDHA enhances HA-specific antibody production without the use of classic adjuvants
A) C57Bl/6 mice were immunized i.m. at week 0, 2 and 4 with PBS (mock), A/California/04/2009 recombinant HA plus vehicle control (vehicle) or with A/California/04/2009 recombinant HA plus 1 μg 17-HDHA (17-HDHA). HA-specific IgM and HA-specific IgG antibody levels were measured by ELISA at B–C) week 2, DE) week 4 and F–G) week 6 (n=10/group). Data shown as mean ±SEM. Statistical analysis done using one-way ANOVA (*p≤0.05, **p≤0.01, ***p≤0.001).
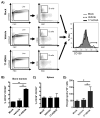
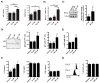
Figure 4. 17-HDHA enhances plasma cell frequency in the bone marrow
C57Bl/6 mice were immunized i.m. with PBS (mock), A/California/04/2009 HA plus vehicle control (vehicle) or A/California/04/2009 HA plus 17-HDHA (17-HDHA). Six weeks later, bone marrow and spleen were collected. Cells were isolated and stained for flow cytometry analysis (n=10/group). A) Gating strategy (left side) and representative histogram of CD138 expression on CD19+ bone marrow B cells (right side). Quantification of B) bone marrow plasma cells (CD19+ CD138+) and C) spleen plasma cells (CD19+ CD138+). D) Quantification of HA-specific IgG secreting bone marrow cells. Analysis done by ELISpot (n=6/groups). Data shown as mean ±SEM. Statistical analysis done using one-way ANOVA (*p≤0.05, ***p≤0.001).
Figure 5. 17-HDHA-mediated HA-specific antibodies are protective against live influenza infection
C57Bl/6 mice were immunized i.m. with PBS (mock), A/California/04/2009 HA plus vehicle control (vehicle), or A/California/04/2009 HA plus 17-HDHA (17-HDHA). A) Neutralizing antibody titers in mice. Serum from mice collected 14 days post final vaccination was used in a GFP microneutralization assay. The neutralization titer was determined as the highest dilution providing >50% reduction in GFP expression from triplicate wells. Columns represent the geometric mean neutralization titer from nine mice. A dotted line represents the limit of detection (<20) for antigen-specific antibodies. Negative samples were arbitrarily assigned a value of 10. The number of mice that had a microneutralization titer equal to or greater than 40 is shown in parentheses. B–C) Twenty-eight days after the last immunization, mice were infected intra-nasally with 300 infectious units of H1N1 influenza virus (A/California/04/E3/2009) (n=10/group). B) Weight and C) survival were monitored daily for 14 days following infection. Results expressed as mean ± SEM. For weight loss analysis, statistical analysis was performed by calculating the area under the curve followed by a Chi-square test (***p≤0.001). Survival rates were analyzed by doing a log-rank (Mantel-Cox) test (*p≤0.05, **p≤0.01). D) Direct antiviral activity of 17-HDHA. MDCK cells were infected with pH1N1/E3 at an MOI of 0.001 for 48 hours in the presence of 5-fold serial dilutions of 17-HDHA, oseltamivir or vehicle control (<0.2% v/v; dotted line). Virus titers in triplicate wells were determined by immunofocus assay. Results expressed as mean ± SEM. Significance was determined against vehicle using unpaired two-tailed Student’s t test (***P≤0.001, **P≤0.01).
Figure 6. 17-HDHA increases B cell expression of CD80 and CD86, but not MHC class II
CD19+B cells were isolated from the spleens of naïve C57Bl/6 mice (n=6). Purified B cells were cultured and stimulated with CpG 1826 ODN (1 μg/ml) plus anti-IgM (2 μg/ml). Cells were treated with 17-HDHA or vehicle control for 30 minutes prior to B cell activation and treatments were repeated daily for 6 days. Cells were collected and stained for A) CD80, B) CD86 and C) MHC class II, and analyzed by flow cytometry at day 6. Results showed mean fluorescence intensity (MFI) ± SEM. Statistical analysis was performed using a one-way ANOVA with a Bonferroni post-test (*p≤0.05, **p≤0.01, ***p≤0.001).
Figure 7. 17-HDHA increases B cell antibody production and promotes B cell differentiation towards an antibody-secreting B cell phenotype
A–C) CD19+B cells were isolated from the spleens of naïve C57Bl/6 mice (n=6). Purified B cells were cultured in triplicate and stimulated with CpG 1826 ODN (1 μg/ml) plus anti-IgM (2 μg/ml). Cells were treated with 17-HDHA at 10 nM and 100 nM, or vehicle control for 30 minutes prior to B cell activation. 17-HDHA or vehicle control treatments were added daily for up to 6 days. Culture supernatants were collected and A) IgM and IgG were measured by ELISA. B) Blimp-1 steady levels were measured by real-time quantitative PCR and normalized to 7S expression. C) Blimp-1 protein levels were measured by Western blot, representative blot images (left panels) and densitometry (right panels) are shown. D–H) Purified CD19+B cells isolated from naïve mouse spleens were depleted of plasma cells (CD19+ CD138+) via FACS (n=6). CD19+ CD138− B cells were then stimulated with CpG 1826 ODN plus anti-IgM. D) IgM and IgG-secreting cells were quantified after 5 days of activation using ELISpot analysis. E) Plasmablasts (IgD− IgM− IgG−) differentiation was measured by flow cytometry after 6 days of activation. F) Supernatants were collected from 6 day activated cultures and IL-10, IL-6and TNFα levels were measured by ELISA.G) The percent of dividing cells was measured using CFSE tracing and flow cytometry analysis. Representative histogram (left panel) and quantification of dividing cells (right panel) are shown. Results showed as mean ± SEM. Statistical analysis was performed using a one-way ANOVA with a Bonferroni post-test (*p≤0.05, **p≤0.01, ***p≤0.001).
Similar articles
- Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands Work Additively via MyD88 To Induce Protective Antiviral Immunity in Mice.
Goff PH, Hayashi T, He W, Yao S, Cottam HB, Tan GS, Crain B, Krammer F, Messer K, Pu M, Carson DA, Palese P, Corr M. Goff PH, et al. J Virol. 2017 Sep 12;91(19):e01050-17. doi: 10.1128/JVI.01050-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724768 Free PMC article. - Influence of adjuvants on the amount, specificity and functional activity of antibody response to human influenza vaccine in mice.
Voutssas-Lara J, Cervantes-Torres J, Hernández M, Bobes RJ, Lamoyi E, Vázquez-Ramírez RA, Mendoza L, Reyes-Barrera KL, López-Martínez R, Alpuche-Solís ÁG, Rosales-Mendoza S, Huerta L, Fragoso G, Sciutto E. Voutssas-Lara J, et al. Mol Immunol. 2021 Jul;135:398-407. doi: 10.1016/j.molimm.2021.05.003. Epub 2021 May 20. Mol Immunol. 2021. PMID: 34022515 - Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.
Zhang H, Zheng H, Guo P, Hu L, Wang Z, Wang J, Ju Y, Meng S. Zhang H, et al. J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article. - Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine.
Nagashima KA, Mousa JJ. Nagashima KA, et al. Viruses. 2021 Mar 24;13(4):546. doi: 10.3390/v13040546. Viruses. 2021. PMID: 33805245 Free PMC article. Review. - Studies on the usefulness of intranasal inactivated influenza vaccines.
Tamura S. Tamura S. Vaccine. 2010 Aug 31;28(38):6393-7. doi: 10.1016/j.vaccine.2010.05.019. Epub 2010 May 20. Vaccine. 2010. PMID: 20493820 Review.
Cited by
- Profiling lipid mediators in serum from children with H1N1 influenza.
Chen W, Gu Y, Ma Y, Dong L, Pan L, Ji C, Guo L, Qi L, Zhang Y, Gao F. Chen W, et al. Sci Rep. 2024 Jul 2;14(1):15186. doi: 10.1038/s41598-024-66190-y. Sci Rep. 2024. PMID: 38956313 Free PMC article. - Emerging mechanisms of obesity-associated immune dysfunction.
Shaikh SR, Beck MA, Alwarawrah Y, MacIver NJ. Shaikh SR, et al. Nat Rev Endocrinol. 2024 Mar;20(3):136-148. doi: 10.1038/s41574-023-00932-2. Epub 2023 Dec 21. Nat Rev Endocrinol. 2024. PMID: 38129700 Review. - Effect of stretching on inflammation in a subcutaneous carrageenan mouse model analyzed at single-cell resolution.
Berrueta L, Muñoz-Vergara D, Martin D, Thompson R, Sansbury BE, Spite M, Badger GJ, Langevin HM. Berrueta L, et al. J Cell Physiol. 2023 Dec;238(12):2778-2793. doi: 10.1002/jcp.31133. Epub 2023 Nov 1. J Cell Physiol. 2023. PMID: 37909412 - Respiratory viral infection and resolution of inflammation: Roles for specialized pro-resolving mediators.
Tavares LP, Nijmeh J, Levy BD. Tavares LP, et al. Exp Biol Med (Maywood). 2023 Oct;248(19):1635-1644. doi: 10.1177/15353702231199082. Epub 2023 Oct 14. Exp Biol Med (Maywood). 2023. PMID: 37837390 Free PMC article. Review. - Investigating the catalytic efficiency of C22-Fatty acids with LOX human isozymes and the platelet response of the C22-oxylipin products.
Tran M, Stanger L, Narendra S, Holinstat M, Holman TR. Tran M, et al. Arch Biochem Biophys. 2023 Oct 1;747:109742. doi: 10.1016/j.abb.2023.109742. Epub 2023 Sep 9. Arch Biochem Biophys. 2023. PMID: 37696384 Free PMC article.
References
- Administration, U. S. F. a. D. Common Ingredients in U.S. Licensed Vaccines. 2013 http://www.fda.gov.
- Prevention, C. f. D. C. a. Lessons from a Virus: Public Health Laboratories Respond to the H1N1 Pandemic. Center for Disease Control and Prevention; 2012. CDC.gov.
- Treanor JJ, El Sahly H, King J, Graham I, Izikson R, Kohberger R, Patriarca P, Cox M. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok(R)) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011;29:7733–7739. - PubMed
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, Cox NJ. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2010;59:1–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI103690/AI/NIAID NIH HHS/United States
- ES01247/ES/NIEHS NIH HHS/United States
- R21NS075611/NS/NINDS NIH HHS/United States
- GM038765/GM/NIGMS NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
- R21 NS075611/NS/NINDS NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
- R01 GM038765/GM/NIGMS NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- T32 AI007285/AI/NIAID NIH HHS/United States
- R03 AI099681/AI/NIAID NIH HHS/United States
- AI103690/AI/NIAID NIH HHS/United States
- T90 DE021985/DE/NIDCR NIH HHS/United States
- R01 AI077719/AI/NIAID NIH HHS/United States
- R03AI099681/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources