Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons - PubMed (original) (raw)
Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons
Mathilde Chivet et al. J Extracell Vesicles. 2014.
Abstract
Exosomes are nano-sized vesicles of endocytic origin released into the extracellular space upon fusion of multivesicular bodies with the plasma membrane. Exosomes represent a novel mechanism of cell-cell communication allowing direct transfer of proteins, lipids and RNAs. In the nervous system, both glial and neuronal cells secrete exosomes in a way regulated by glutamate. It has been hypothesized that exosomes can be used for interneuronal communication implying that neuronal exosomes should bind to other neurons with some kind of specificity. Here, dissociated hippocampal cells were used to compare the specificity of binding of exosomes secreted by neuroblastoma cells to that of exosomes secreted by cortical neurons. We found that exosomes from neuroblastoma cells bind indiscriminately to neurons and glial cells and could be endocytosed preferentially by glial cells. In contrast, exosomes secreted from stimulated cortical neurons bound to and were endocytosed only by neurons. Thus, our results demonstrate for the first time that exosomes released upon synaptic activation do not bind to glial cells but selectively to other neurons suggesting that they can underlie a novel aspect of interneuronal communication.
Keywords: CD63 tetraspanin; central nervous system; exosomes; extracellular vesicles; intercellular communication; multivesicular bodies; neurons; tetanus toxin.
Figures
Fig. 1
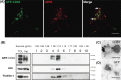
N2a cells constitutively expressing GFP-CD63 (N2aGFP-CD63) secrete exosomes containing the fusion protein. (A) Co-staining of N2aGFP-CD63 with anti-LBPA (red) shows that GFP-CD63 (green) is concentrated inside LBPA-containing multivesicular bodies (arrows). (B) Density separation of extracellular vesicles released by N2aGFP-CD63 cells; Western blot analysis using anti-GFP, anti-flotillin-1 and anti-Alix, shows GFP immunoreactivity in fractions containing exosomes. TCL: total cell lysates, Inp: input. (C, D) Immunogold labelling of vesicles secreted by N2aGFP-CD63 cells pelleted at 100,000×g. Anti-CD63 labels the surface of intact exosomes (C), while anti-GFP stains the lumen of permeabilized exosomes (D). Scale bars: (A) 5 µm, (C, D) 100 nm.
Fig. 2
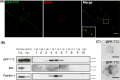
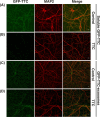
Soluble GFP–TTC is endocytosed before being secreted via neuronal exosomes. Cortical neurons were incubated at 37°C with GFP–TTC for 2 h (36 nM). (A) Confocal microscopy of neurons stained with anti-EEA1 (red), shows that GFP–TTC proteins (green) bind to and are endocytosed by neurons. (B) Density separation of extracellular vesicles secreted during a 15 min treatment with bicuculline and 4-AP; Western blot analysis using anti-GFP, anti-flotillin-1 and anti-Alix, shows GFP immunoreactivity in fractions containing exosomes. TCL: total cell lysates, Inp: input. (C) Immunogold labelling with anti-GFP of vesicles pelleted at 100,000×g demonstrates the presence of GFP–TTC on the exosomal surface. Scale bars: (A) 10 µm (C) 100 nm.
Fig. 3
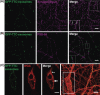
Neuroblastoma exosomes carrying GFP-CD63 bind to and are endocytosed by neurons and glial cells. Exosomes released by N2aGFP-CD63 cells were resuspended in conditioned medium and incubated for 1 h on mixed primary culture of hippocampal neurons (14 DIV). After washing, cells were co-stained with antibodies against MAP2 (A), or GFAP (B), or O4 (C), to label neurons, astrocytes and oligodendrocytes, respectively, and with antibodies against Lamp-1 (A, B, C) to stain late endosomes and lysosomes. Arrows point to exosomes bound on the cell surface. Arrowheads point to colocalization of exosomes with Lamp-1. Stacks of 3 confocal sections inside cells are shown for all conditions. Scale bars: 10 µm.
Fig. 4
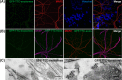
Neuronal exosomes bearing GFP–TTC bind specifically to neurons. (A) Exosomes released by cortical neurons pre-incubated with GFP–TTC were harvested, pelleted at 100,000×g and separated on a sucrose gradient. Three GFP–TTC containing fractions (sucrose density of 1.1–1.15 g/ml) were pooled, pelleted, resuspended in incubation medium and incubated for 1 h on hippocampal cell cultures (16 DIV) (A, B, C). A) After washing, cells were immunostained with anti-MAP2 antibody (red) to label neurons. Hoechst nuclear staining shows the presence of numerous MAP2 negative cells which are not stained with GFP–TTC exosomes (maxima intensity). (B) After washing, cells were immunostained with anti-MAP2 antibody (red) to label neurons and anti-GFAP to stain astrocytes (magenta). (C) Cells were washed and processed for immunogold labelling using anti-GFP and processed for EM observation. Single or aggregated exosomes carrying gold-labelled GFP–TTC can be seen on the surface of neurons. Scale bars: (A, B) 10 µm, (C) 500 nm.
Fig. 5
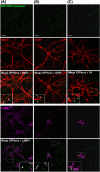
Neuronal exosomes bearing GFP–TTC preferentially bind to pre-synaptic terminals and can be internalized. Exosomes secreted by cortical neurons preincubated with GFP–TTC (green) were incubated for 1 h on hippocampal neurons (22 DIV), which were then immunostained with (A) anti-synaptophysin (magenta) or (B) anti-PSD95 (magenta). Confocal microscopy sections show patches of GFP–TTC exosomes (green) perfectly colocalized with some synaptophysin-positive presynaptic sites and juxtaposed with some PSD95-positive post-synaptic sites. Scale bars: (A, B, C) 10 µm. (C) GFP–TTC exosomes were incubated for 1 h on hippocampal neurons (21 DIV) preincubated with Alexa594-WGA for 10 min at 37°C to label endosomes. (A), (B) (C) are confocal sections except for the photo on the right of the (C) panel, representing the projection of maximal intensities. Thin and bold arrows show labelling on the surface and within the cytoplasm respectively.
Fig. 6
GFP–TTC exosomes do not bind to the neuronal surface because of their TTC cargo. (A, B) Incubation with TTC abolishes GFP–TTC staining of neurons: GFP–TTC was diluted to 0.36 nM in culture medium and incubated for 1 h on 16 DIV hippocampal neurons in absence (A) or in presence (B) of 100 nM TTC. In B) cells were pre-incubated for 20 min with 100 nM TTC. (C, D) TTC does not impair binding of GFP–TTC-exosomes to neurons: GFP–TTC-exosomes were incubated for 1 h on 16 DIV hippocampal neurons in absence (C) or in presence (D) of 100 nM TTC. In D) cells were pre-incubated for 20 min with 100 nM TTC. After incubation, cells were washed, fixed and immunolabelled with anti-MAP2 (red) (A, B, C and D). Scale bars: 10 µm.
Similar articles
- Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity.
Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Lachenal G, et al. Mol Cell Neurosci. 2011 Feb;46(2):409-18. doi: 10.1016/j.mcn.2010.11.004. Epub 2010 Nov 25. Mol Cell Neurosci. 2011. PMID: 21111824 - Purification and Analysis of Exosomes Released by Mature Cortical Neurons Following Synaptic Activation.
Laulagnier K, Javalet C, Hemming FJ, Sadoul R. Laulagnier K, et al. Methods Mol Biol. 2017;1545:129-138. doi: 10.1007/978-1-4939-6728-5_9. Methods Mol Biol. 2017. PMID: 27943211 - Live-Imaging Detection of Multivesicular Body-Plasma Membrane Fusion and Exosome Release in Cultured Primary Neurons.
Pescosolido MF, Ouyang Q, Liu JS, Morrow EM. Pescosolido MF, et al. Methods Mol Biol. 2023;2683:213-220. doi: 10.1007/978-1-0716-3287-1_17. Methods Mol Biol. 2023. PMID: 37300778 - Exosomes as a novel way of interneuronal communication.
Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. Chivet M, et al. Biochem Soc Trans. 2013 Feb 1;41(1):241-4. doi: 10.1042/BST20120266. Biochem Soc Trans. 2013. PMID: 23356290 Review. - Membrane-bound extracellular vesicles secreted by parasitic protozoa: cellular structures involved in the communication between cells.
de Souza W, Barrias ES. de Souza W, et al. Parasitol Res. 2020 Jul;119(7):2005-2023. doi: 10.1007/s00436-020-06691-7. Epub 2020 May 12. Parasitol Res. 2020. PMID: 32394001 Review.
Cited by
- Do extracellular vesicles have specific target cells?; Extracellular vesicle mediated embryo maternal communication.
Dissanayake K, Godakumara K, Muhandiram S, Kodithuwakku S, Fazeli A. Dissanayake K, et al. Front Mol Biosci. 2024 Jul 16;11:1415909. doi: 10.3389/fmolb.2024.1415909. eCollection 2024. Front Mol Biosci. 2024. PMID: 39081929 Free PMC article. - Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets.
Prada I, Meldolesi J. Prada I, et al. Int J Mol Sci. 2016 Aug 9;17(8):1296. doi: 10.3390/ijms17081296. Int J Mol Sci. 2016. PMID: 27517914 Free PMC article. Review. - Exosome: The "Off-the-Shelf" Cellular Nanocomponent as a Potential Pathogenic Agent, a Disease Biomarker, and Neurotherapeutics.
Ghosh S, Ghosh S. Ghosh S, et al. Front Pharmacol. 2022 May 24;13:878058. doi: 10.3389/fphar.2022.878058. eCollection 2022. Front Pharmacol. 2022. PMID: 35685643 Free PMC article. Review. - Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases.
Zou Z, Li H, Xu G, Hu Y, Zhang W, Tian K. Zou Z, et al. Int J Nanomedicine. 2023 Aug 21;18:4751-4778. doi: 10.2147/IJN.S417422. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37635911 Free PMC article. Review. - Extracellular Vesicle as a Source of Alzheimer's Biomarkers: Opportunities and Challenges.
Lee S, Mankhong S, Kang JH. Lee S, et al. Int J Mol Sci. 2019 Apr 8;20(7):1728. doi: 10.3390/ijms20071728. Int J Mol Sci. 2019. PMID: 30965555 Free PMC article. Review.
References
- Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci. 1997;110:1867–77. - PubMed
- Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237–43. - PubMed
- Krämer-Albers E-M, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin Appl. 2007;1:1446–61. - PubMed
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–58. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous