Glycogen metabolism and the homeostatic regulation of sleep - PubMed (original) (raw)
Review
Glycogen metabolism and the homeostatic regulation of sleep
Jean-Marie Petit et al. Metab Brain Dis. 2015 Feb.
Abstract
In 1995 Benington and Heller formulated an energy hypothesis of sleep centered on a key role of glycogen. It was postulated that a major function of sleep is to replenish glycogen stores in the brain that have been depleted during wakefulness which is associated to an increased energy demand. Astrocytic glycogen depletion participates to an increase of extracellular adenosine release which influences sleep homeostasis. Here, we will review some evidence obtained by studies addressing the question of a key role played by glycogen metabolism in sleep regulation as proposed by this hypothesis or by an alternative hypothesis named "glycogenetic" hypothesis as well as the importance of the confounding effect of glucocorticoïds. Even though actual collected data argue in favor of a role of sleep in brain energy balance-homeostasis, they do not support a critical and direct involvement of glycogen metabolism on sleep regulation. For instance, glycogen levels during the sleep-wake cycle are driven by different physiological signals and therefore appear more as a marker-integrator of brain energy status than a direct regulator of sleep homeostasis. In support of this we provide evidence that blockade of glycogen mobilization does not induce more sleep episodes during the active period while locomotor activity is reduced. These observations do not invalidate the energy hypothesis of sleep but indicate that underlying cellular mechanisms are more complex than postulated by Benington and Heller.
Figures
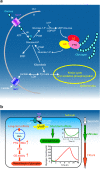
Fig. 1
Gylcogen metabolism in astrocytes. a Schematic representation of glucose metabolism. Glucose enters cells trough glucose transporters (GLUT) and is phosphorylated by hexokinase (HK) to produce glucose-6-phosphate. Glucose 6-phosphate can be processed into different metabolic pathways. It can be metabolized through the pentose phosphate pathway (PPP) or through glycolysis giving rise to pyruvate production. Pyruvate can enter mitochodria where it is metabolized through the Krebs cycle and oxidative phosphorylation. Alternatively, pyruvate can be reduced to lactate by lactate dehydrogenase (LDH) and be released in the extracellular space through monocarboxylate transporters (MCT). Glucose-6-phosphate can also be used to store glucosyl units as glycogen. As a first step glucose-6-phosphate is converted to UDP-glucose through the action of phosphoglucomutase (PGM) and UDP-glucose pyrophosphorylase (UDPGP). UDP-glucose is then incorporated into glycogen by glycogen synthase (GS). Protein targeting to glycogen (PTG) is a specific glycogen-binding G-subunit, which is responsible for the targeting of protein phosphatase 1 to glycogen. Through this action, PTG promotes GS dephosphorylation and activation therefore favouring glycogen synthesis. In case of energy needs, glycogen can be broken down by glycogen phosphorylase (GP) to produce glucose-1-phosphate that is converted back to glucose-6-phosphate through the action of PGM. Adapted from (Allaman 2009) with permission. b Differential modulation of glycogen metabolism in cultured astrocytes by noradrenaline (NA). Activation of cAMP-dependent intracellular signalling by NA results in a short term (seconds to minutes, in green) glycogenolysis and in a delayed (hours, in red) glycogen resynthesis. This long-term response requires induction of gene expression and is accompanied by stimulation of mRNA expression of protein targeting to glycogen (PTG), a member of the glycogen-targeting subunits of protein phosphatase 1, and by the activation of glycogen synthase (see text for details). Adapted from (Magistretti 2006) with permission
Fig. 2
Sleep architecture. a Twenty four hour EEG power spectra in NREM sleep (SWS), REM sleep (PS) and waking in one representative adult male rat (Wistar Kyoto strain). Slow Wave Activity (SWA) frequency spectrum (1–4.5 Hz) is highlighted in grey. b EEG signals and corresponding cortical multiunit activity (raster plots below; each bar is a spike) representative of the three vigilance states. Note the fast irregular pattern of cortical firing in waking and REM sleep, and regular occurrence of generalized neuronal silence, corresponding to EEG slow waves, in NREM sleep. UP (red) and DOWN (blue) states during NREM sleep are highlighted. Adapted from (Vyazovskiy and Faraguna 2014) with permission
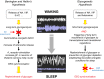
Fig. 3
Energy hypotheses of sleep proposing glycogen as key regulator of sleep homeostasis. Schematic views of Benington and Heller’s hypothesis (left side) and the “glycogenetic” hypothesis (right side) are shown (see text for detailed description). Red arrows indicate steps that are not supported by experimental data. NA: noradrenaline; VIP: vasoactive intestinal peptide; 5-HT: 5-hydroxytryptamine or serotonin; SD: sleep deprivation; DAB: 1,4-dideoxy-1,4-imino-d-arabinitol; EEG: electroencephalogram; EMG: electromyogram
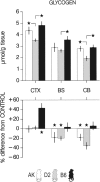
Fig. 4
Changes in brain glycogen after sleep deprivation vary with genotype. Brain glycogen content in sleep-deprived mice. Brain glycogen varied with strain and structure (2-way ANOVA with repeated measures for factor structure. Factor strain, F 2,30 = 13.2, P < 0.0001; structure, F 2,60 = 71.9, P < 0.0001; interaction, F 4,60 = 1.6, P = 0.20). Bars represent mean values ±2SE. Top panels: *significant strain differences for each brain region (Tukey’s range test; P < 0.05). Bottom panels: *significant differences from control (unpaired 2-sided _t_-tests; P < 0.05). In the bottom panels, values were expressed as % difference from the mean values in the control group (0 %). CTX, cerebral cortex; BS, brainstem; CB, cerebellum. Adapted from (Franken et al. 2003) with permission
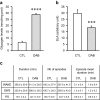
Fig. 5
Effects of acute pharmacological inhibition of glycogen mobilization on locomotor activity and sleep parameters when added at dark onset. Experiments were performed on adult males mice (C57BL/6j from Janvier, France) equipped with EEG and EMG electrodes (for further details see Petit et al. 2013) and implanted with a chronic cannula into the lateral ventricle. The mice were housed individually under a 12-h:12-h light–dark cycle at 23 °C ambient temperature with food and water ad libitum. Two weeks after the surgery, they were injected with 2 μl of NaCl 0.9 % (control [CTL] group, white bars) or 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) at 0.5 M dissolved in NaCl 0.9 % (DAB group, gray bars) at the beginning of the dark period (Zeitgeber Time 12, ZT12) under a light isoflurane anesthesia. After injections, mice were let 2 h in their cages for anesthesia recovery before recordings begin. a Brain glycogen levels 3 h after i.c.v. administration of DAB. After the injection, the animals were replaced in their cages and sacrificed 3 h later (ZT15). The brains were quickly removed, frozen and cut for site injection verification and standard glycogen dosage (Petit et al. 2010). The values represent the mean of glycogen levels in nmol/mg prot (±SEM) measured on brain slices from -200 μm posterior to +200 μm anterior compared to the injection site in both group (CTL vs DAB). N = 6. Statistics: Unpaired t test with Welch’s correction,**** p value <0.0001 compared to CTL. b Spontaneous Locomotor Activity (SLA) after i.c.v injection of DAB. Each cage was equipped with passive infrared sensors on the top for SLA recordings. SLA was recorded for 10-min interval during 10 h from ZT14 to ZT24. The same animals received NaCl 0.9 % and DAB on separate days. The values represent mean SLA ± SEM over this 10 h period in both group (CTL vs DAB). N = 12. Statistics: Paired t test, *** p value = 0.0004 compared to CTL. c Quantitative parameters of the sleep wake cycle after i.c.v. injection of DAB. The EEG/EMG signals were recorded and digitalized with an Embla A10 amplifier (Medcare, USA). The EEG was divided in 4-s epochs which were visually scored in one of the three states of vigilance (wakefulness (W), slow wave sleep (SWS) or paradoxical sleep (PS)) according to classical criteria (Tobler et al. 1997). Values represent the mean values (±SEM) of cumulative duration (min), number of episodes and episode mean duration (min) for each state of vigilance for the 10 h of recording session from ZT14 to ZT24. N = 6 animals in each group. Statistics: One way ANOVA followed by Bonferroni’s post-test. No statistical difference was observed between the control and the DAB groups
Similar articles
- The energy hypothesis of sleep revisited.
Scharf MT, Naidoo N, Zimmerman JE, Pack AI. Scharf MT, et al. Prog Neurobiol. 2008 Nov;86(3):264-80. doi: 10.1016/j.pneurobio.2008.08.003. Epub 2008 Sep 3. Prog Neurobiol. 2008. PMID: 18809461 Free PMC article. Review. - Contributions of glycogen to astrocytic energetics during brain activation.
Dienel GA, Cruz NF. Dienel GA, et al. Metab Brain Dis. 2015 Feb;30(1):281-98. doi: 10.1007/s11011-014-9493-8. Epub 2014 Feb 12. Metab Brain Dis. 2015. PMID: 24515302 Free PMC article. Review. - Changes in brain glycogen after sleep deprivation vary with genotype.
Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC. Franken P, et al. Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285(2):R413-9. doi: 10.1152/ajpregu.00668.2002. Epub 2003 May 1. Am J Physiol Regul Integr Comp Physiol. 2003. PMID: 12730076 - Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep.
Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Kong J, et al. J Neurosci. 2002 Jul 1;22(13):5581-7. doi: 10.1523/JNEUROSCI.22-13-05581.2002. J Neurosci. 2002. PMID: 12097509 Free PMC article. - Effects of diabetes on brain metabolism--is brain glycogen a significant player?
Sickmann HM, Waagepetersen HS. Sickmann HM, et al. Metab Brain Dis. 2015 Feb;30(1):335-43. doi: 10.1007/s11011-014-9546-z. Epub 2014 Apr 29. Metab Brain Dis. 2015. PMID: 24771109 Review.
Cited by
- Three-dimensional immersive virtual reality for studying cellular compartments in 3D models from EM preparations of neural tissues.
Calì C, Baghabra J, Boges DJ, Holst GR, Kreshuk A, Hamprecht FA, Srinivasan M, Lehväslaiho H, Magistretti PJ. Calì C, et al. J Comp Neurol. 2016 Jan 1;524(1):23-38. doi: 10.1002/cne.23852. Epub 2015 Aug 11. J Comp Neurol. 2016. PMID: 26179415 Free PMC article. - Effects of Alcohol Extracts From Ganoderma resinaceum on Sleep in Mice Using Combined Transcriptome and Metabolome Analysis.
Chen T, Zhang F, Chen J, Zhong Q, Hu Y, Wu R, Xie B, Jiang Y, Chen B. Chen T, et al. Front Nutr. 2022 Jan 28;9:745624. doi: 10.3389/fnut.2022.745624. eCollection 2022. Front Nutr. 2022. PMID: 35165654 Free PMC article. - Fatigue, Sleep, and Autoimmune and Related Disorders.
Zielinski MR, Systrom DM, Rose NR. Zielinski MR, et al. Front Immunol. 2019 Aug 6;10:1827. doi: 10.3389/fimmu.2019.01827. eCollection 2019. Front Immunol. 2019. PMID: 31447842 Free PMC article. Review. - AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance.
Nagy S, Maurer GW, Hentze JL, Rose M, Werge TM, Rewitz K. Nagy S, et al. PLoS Genet. 2018 Dec 19;14(12):e1007623. doi: 10.1371/journal.pgen.1007623. eCollection 2018 Dec. PLoS Genet. 2018. PMID: 30566533 Free PMC article. - Glycogen Repletion in Brown Adipose Tissue upon Refeeding Is Primarily Driven by Phosphorylation-Independent Mechanisms.
Carmean CM, Huang YH, Brady MJ. Carmean CM, et al. PLoS One. 2016 May 23;11(5):e0156148. doi: 10.1371/journal.pone.0156148. eCollection 2016. PLoS One. 2016. PMID: 27213961 Free PMC article.
References
- Allaman I. Glial Glycogen Metabolism. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford: Academic; 2009. pp. 811–818.
- Allaman I, Pellerin L, Magistretti PJ. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia. 2000;30:382–391. - PubMed
- Allaman I, Lengacher S, Magistretti PJ, Pellerin L. A2B receptor activation promotes glycogen synthesis in astrocytes through modulation of gene expression. Am J Physiol Cell Physiol. 2003;284:C696–C704. - PubMed
- Allaman I, Pellerin L, Magistretti PJ. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem. 2004;88:900–908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources