Angiotensin blockade in late autosomal dominant polycystic kidney disease - PubMed (original) (raw)
Randomized Controlled Trial
. 2014 Dec 11;371(24):2267-76.
doi: 10.1056/NEJMoa1402686. Epub 2014 Nov 15.
Kaleab Z Abebe, Arlene B Chapman, Robert W Schrier, William E Braun, Theodore I Steinman, Franz T Winklhofer, Godela Brosnahan, Peter G Czarnecki, Marie C Hogan, Dana C Miskulin, Frederic F Rahbari-Oskoui, Jared J Grantham, Peter C Harris, Michael F Flessner, Charity G Moore, Ronald D Perrone; HALT-PKD Trial Investigators
Collaborators, Affiliations
- PMID: 25399731
- PMCID: PMC4284824
- DOI: 10.1056/NEJMoa1402686
Randomized Controlled Trial
Angiotensin blockade in late autosomal dominant polycystic kidney disease
Vicente E Torres et al. N Engl J Med. 2014.
Abstract
Background: Hypertension develops early in patients with autosomal dominant polycystic kidney disease (ADPKD) and is associated with disease progression. The renin-angiotensin-aldosterone system (RAAS) is implicated in the pathogenesis of hypertension in patients with ADPKD. Dual blockade of the RAAS may circumvent compensatory mechanisms that limit the efficacy of monotherapy with an angiotensin-converting-enzyme (ACE) inhibitor or angiotensin II-receptor blocker (ARB).
Methods: In this double-blind, placebo-controlled trial, we randomly assigned 486 patients, 18 to 64 years of age, with ADPKD (estimated glomerular filtration rate [GFR], 25 to 60 ml per minute per 1.73 m(2) of body-surface area) to receive an ACE inhibitor (lisinopril) and placebo or lisinopril and an ARB (telmisartan), with the doses adjusted to achieve a blood pressure of 110/70 to 130/80 mm Hg. The composite primary outcome was the time to death, end-stage renal disease, or a 50% reduction from the baseline estimated GFR. Secondary outcomes included the rates of change in urinary aldosterone and albumin excretion, frequency of hospitalizations for any cause and for cardiovascular causes, incidence of pain, frequency of ADPKD-related symptoms, quality of life, and adverse study-medication effects. Patients were followed for 5 to 8 years.
Results: There was no significant difference between the study groups in the incidence of the composite primary outcome (hazard ratio with lisinopril-telmisartan, 1.08; 95% confidence interval, 0.82 to 1.42). The two treatments controlled blood pressure and lowered urinary aldosterone excretion similarly. The rates of decline in the estimated GFR, urinary albumin excretion, and other secondary outcomes and adverse events, including hyperkalemia and acute kidney injury, were also similar in the two groups.
Conclusions: Monotherapy with an ACE inhibitor was associated with blood-pressure control in most patients with ADPKD and stage 3 chronic kidney disease. The addition of an ARB did not alter the decline in the estimated GFR. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; HALT-PKD [Study B] ClinicalTrials.gov number, NCT01885559.).
Figures
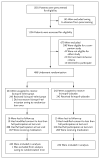
Figure 1. Enrollment, Study-Group Assignments, and Follow-up
We screened 1156 patients, 486 of whom were randomly assigned to receive lisinopril–telmisartan or lisinopril–placebo. Overall, 426 participants completed the trial according to the protocol (i.e., full participation), and 5.8% of the patients discontinued the study medication, reduced the number of study visits or assessments, or both (i.e., modified consent to less than full participation). All but 1 participant who was ineligible for randomization (485 participants) were included in the analysis of the primary outcome.
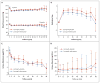
Figure 2. Blood-Pressure Levels, Medication Steps, and Urinary Aldosterone and Albumin Excretion
The graphs show the mean systolic and diastolic blood pressures (Panel A), steps in the medication-dose adjustments (Panel B, and Table S1 in the Supplementary Appendix), urinary aldosterone excretion (Panel C), and median urinary albumin excretion (Panel D) in participants treated with lisinopril–telmisartan and those who received lisinopril–placebo. I bars indicate 95% confidence intervals (Panels A, B, and C) or interquartile ranges (Panel D).
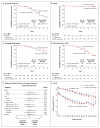
Figure 3. Effect of Lisinopril–Telmisartan, as Compared with Lisinopril–Placebo, on the Time to Primary-Outcome Events and on the Estimated Glomerular Filtration Rate (eGFR)
The graphs show the probability of freedom from the composite outcome (Panel A) and its components: death (Panel B), end-stage renal disease (Panel C), and a 50% reduction from the baseline eGFR (Panel D). Panel E shows the hazard ratios for the primary composite outcome for the lisinopril–telmisartan group, as compared with the lisinopril–placebo group, according to prespecified subgroups. The P values are for the interaction of subgroup by treatment. Horizontal bars indicate 95% confidence intervals. Panel F shows the mean eGFR values at baseline, after 4 months of treatment, and yearly thereafter from 12 to 96 months after the start of treatment for all participants in the two treatment groups who either had an end point or withdrew from the study (diamonds) and for those who completed the study follow-up (circles). I bars represent 95% confidence intervals. The change in the eGFR over time for participants who completed the study follow-up is linear. The appearance of a deceleration in the rate of decline of the eGFR for participants who met an end point or withdrew is probably due to the early attrition of participants with rapid disease progression.
Comment in
- A quest--halting the progression of autosomal dominant polycystic kidney disease.
Ellison DH, Ingelfinger JR. Ellison DH, et al. N Engl J Med. 2014 Dec 11;371(24):2329-31. doi: 10.1056/NEJMe1412586. Epub 2014 Nov 15. N Engl J Med. 2014. PMID: 25399732 No abstract available.
References
- Grantham JJ. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–85. - PubMed
- Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2009;20:1888–93. - PubMed
- Graham PC, Lindop GB. The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 1988;33:1084–90. - PubMed
- Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin–angiotensin–aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–6. - PubMed
- Torres VE, Wilson DM, Burnett JCJ, Jr, Johnson CM, Offord KP. Effect of inhibition of converting enzyme on renal hemodynamics and sodium management in polycystic kidney disease. Mayo Clin Proc. 1991;66:1010–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK062401/DK/NIDDK NIH HHS/United States
- TR001064/TR/NCATS NIH HHS/United States
- P30 DK090728/DK/NIDDK NIH HHS/United States
- U01 DK062402/DK/NIDDK NIH HHS/United States
- TR001102/TR/NCATS NIH HHS/United States
- M01 RR001032/RR/NCRR NIH HHS/United States
- RR000585/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- RR025752/RR/NCRR NIH HHS/United States
- M01 RR000585/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- DK62401/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- DK62410/DK/NIDDK NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- RR033179/RR/NCRR NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- M01 RR000039/RR/NCRR NIH HHS/United States
- UL1 RR033179/RR/NCRR NIH HHS/United States
- DK62402/DK/NIDDK NIH HHS/United States
- TR000001/TR/NCATS NIH HHS/United States
- TR00135/TR/NCATS NIH HHS/United States
- M01 RR000054/RR/NCRR NIH HHS/United States
- M01 RR023940/RR/NCRR NIH HHS/United States
- RR024989/RR/NCRR NIH HHS/United States
- DK62408/DK/NIDDK NIH HHS/United States
- RR000054/RR/NCRR NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- U01 DK062408/DK/NIDDK NIH HHS/United States
- RR025758/RR/NCRR NIH HHS/United States
- TR001082/TR/NCATS NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- RR001032/RR/NCRR NIH HHS/United States
- TR000454/TR/NCATS NIH HHS/United States
- U01 DK062411/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- RR023940/RR/NCRR NIH HHS/United States
- U01 DK082230/DK/NIDDK NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- RR000039/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- DK082230/DK/NIDDK NIH HHS/United States
- RR024150/RR/NCRR NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- RR025780/RR/NCRR NIH HHS/United States
- TR000439/TR/NCATS NIH HHS/United States
- DK62411/DK/NIDDK NIH HHS/United States
- U01 DK062410/DK/NIDDK NIH HHS/United States
- RR025008/RR/NCRR NIH HHS/United States
- RR000051/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous