Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy - PubMed (original) (raw)
. 2014 Dec 2;111(48):17266-71.
doi: 10.1073/pnas.1419599111. Epub 2014 Nov 19.
Bing Cui 1, Hsien Lai 1, Grace Liu 1, Emanuela M Ghia 1, George F Widhopf 2nd 1, Zhuhong Zhang 1, Christina C N Wu 1, Liguang Chen 1, Rongrong Wu 1, Richard Schwab 1, Dennis A Carson 2, Thomas J Kipps 2
Affiliations
- PMID: 25411317
- PMCID: PMC4260559
- DOI: 10.1073/pnas.1419599111
Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy
Suping Zhang et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Although initially responsive to chemotherapy, many patients with ovarian cancer subsequently develop relapsed and potentially fatal metastatic disease, which is thought to develop from cancer stem cells (CSCs) that are relatively resistant to conventional therapy. Here, we show that CSCs express a type I receptor tyrosine kinase-like orphan receptor (ROR1), which is expressed during embryogenesis and by many different cancers, but not normal postpartum tissues. Ovarian cancers with high levels of ROR1 had stem cell-like gene-expression signatures. Furthermore, patients with ovarian cancers with high levels of ROR1 had higher rates of relapse and a shorter median survival than patients with ovarian cancers that expressed low-to-negligible amounts of ROR1. We found that ROR1-positive (ROR1(+)) cells isolated from primary tumor-derived xenografts (PDXs) also expressed aldehyde dehydrogenase 1 (ALDH1) and had a greater capacity to form spheroids and to engraft immune-deficient mice than did ROR1-negative (ROR1(Neg)) ovarian cancer cells isolated from the same tumor population. Treatment with UC-961, an anti-ROR1 mAb, or shRNA silencing of ROR1 inhibited expression of the polycomb ring-finger oncogene, Bmi-1, and other genes associated with the epithelial-mesenchymal transition. Moreover, shRNA silencing of ROR1, depletion of ROR1(+) cells, or treatment with UC-961 impaired the capacity of ovarian cancer cells to form spheroids or tumor xenografts. More importantly, treatment with anti-ROR1 affected the capacity of the xenograft to reseed a virgin mouse, indicating that targeting ROR1 may affect CSC self-renewal. Collectively, these studies indicate that ovarian CSCs express ROR1, which contributes to their capacity to form tumors, making ROR1 a potential target for the therapy of patients with ovarian cancer.
Keywords: PDX mice model; ROR1; monoclonal antibody; ovarian cancer stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
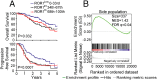
Fig. 1.
Ovarian cancers that express high levels of ROR1 have stem cell-like gene-expression signatures and relatively poor prognosis. (A) Graphs were derived from published data available through the PubMed GEO database (GSE9891). Kaplan–Meier curves depict overall survival (Upper) or progression-free survival (Lower) of patients with ROR1Low (blue line), ROR1Int (black line), or ROR1Hi (red line) ovarian cancers. The P value for the difference between ROR1Low versus ROR1Hi subgroups was determined by the log-rank test. (B) Enrichment plots of side-population gene-expression signatures (23) on ROR1Hi tumors versus ROR1Low cancers in the GSE9891 dataset. Size is the number of genes included in the analysis. NES (normalized enrichment score) accounts for the difference in gene-set size and can be used to compare the analysis results across gene sets. FDR q-val (false discovery rate q value) is the estimated probability that a gene set with a given NES represents a false positive finding. Each gene set is considered significant when the false discovery rate (FDR) is less than 25%. The middle portion of the plot shows where the members of the gene set appear in the list of ranked genes; red and blue colors represent positive and negative correlation with ROR1 expression, respectively.
Fig. 2.
ROR1 is expressed on ovarian cancer cells with high ALDH1 activity. (A) OV1110, AA1581, or AA0857 were stained with either HE (Upper) or with the anti-ROR1 mAb 4A5 (Lower). Bound 4A5 is shown in red. (Scale bar: 35 μm.) (B) Flow-cytometric analysis of OV1110, AA1581, or AA0857. The cells were stained with 4A5 or control mAb, and with ALDOFLUOR without (−) or with (+) the ALDH1 inhibitor DEAB, as indicated at the top. The open boxes in the each contour plot indicate the gates for identifying cells with ALDH1 activity, the proportion of which is indicated. The open boxes in the left of the contour plots depict the gates used to identify cells that are certain to lack ALDH1 activity. In the lower row are histograms depicting the fluorescence of cells within these boxes that were negative (left) or positive (right) for ALDH1 activity. The filled histograms depict the fluorescence of cells stained with an isotype-control mAb whereas the open histograms depict the fluorescence of cells stained with 4A5. The number in each plot provides the mean fluorescence intensity ratio (MFIR).
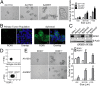
Fig. 3.
ROR1+ cells give rise to more spheroids than ROR1Neg cells. (A) Photomicrographs of spheroids that developed from cultured single cells isolated from OV1110, AA1581, or AA0857. (Scale bar: 100 µm, except for Far Right, which displays the spheroid in the box under higher magnification.) The bar graph to the Right depicts the average numbers of small (<50 µm), medium (50–100 µm), or large (>100 µm) spheroids formed by cells of OV1110 (open), AA1581 (gray), or AA0857 (black) in triplicate wells ± SEM. Asterisks (*) indicate the statistical significance of differences in the number of spheroids of cells from OV1110 versus AA1581 or AA0857 (*P < 0.05, **_P_ < 0.01, using Student’s _t_ test). (_B_) Confocal microscopy of spheroids stained with 4A5 (green). Nuclear staining is in blue. (Scale bar: 10 μm.) (_C_) Immunoblot analyses of cell lysates prepared from cell lines Jeko-1 (ROR1+) or Ramos (ROR1Neg), the primary tumor (Primary), or spheroids, as indicated on the top of each lane; the tumor from which the lysates were derived is indicated at the bottom. The blots were probed for ROR1 or β-Actin, as indicated on the right. The numbers between the panels provide the ratio of band intensities for blots probed with anti-ROR1 versus β-Actin, using Image J software. (_D_) Strategy for sorting ROR1+ versus ROR1Neg cells. The open boxes indicate the gates used to select ROR1Neg (left) or ROR1+ (right) cells. (_E_) Photomicrographs of ROR1+ or ROR1Neg cells isolated from AA1581 (_Upper_) or AA0857 (_Lower_), as indicated on the left margin. (Scale bar: 100 µm.) The bar graph (_Right_) depicts the average numbers of small (<50 µm), medium (50–100 µm), or large (>100 µm) spheroids formed in three separate culture wells containing ROR1+ cells (filled bars) or ROR1Neg cells (open bars), as indicated at the bottom of histograms (n.d., not detectable). Error bars indicate SEM. Asterisks (*) indicate the statistical significance of differences between the number of spheroids that formed by ROR1+ cells versus ROR1Neg cells of AA0857 (Upper) or AA1581 (Lower) (*P < 0.05, **P < 0.01, using Student’s t test). FSC, forward light scatter.
Fig. 4.
ROR1+ ovarian cancer cells more effectively engraft immune-deficient mice. (A) Tumor growth over time resulting from injection of 1 × 106 OV1110 (filled boxes), AA1581 (filled circles), or AA0857 (filled inverted triangles) per mouse. Asterisks indicate the statistical significance of differences between the mean sizes of the tumors that developed in mice engrafted with AA0857 versus OV1110 (black), AA1581 versus OV1110 (black), or AA0857 versus AA1581 (red) (*P < 0.05, **P < 0.01, ***P < 0.001, using Student’s t test, n = 5 for each group). (B) Gating strategy used for sorting ROR1+ versus ROR1Neg cells, as in Fig. 3_D_. (C) Photographs of representative tumors extirpated from mice engrafted with ROR1+ or ROR1Neg ovarian cancer cells of AA0857. (Upper) Representative tumors that formed on the flanks of engrafted mice. (Lower) Ovarian tumors orthotopically implanted with sorted ROR1+ or ROR1Neg cells. (D) The fluorescence of single-cell suspensions of unsorted tumor (Initial Tumor), freshly sorted ROR1+ cells before engraftment (Sorted Cell), or cells isolated from tumors that developed in mice engrafted with ROR1+ cells (Tumor of ROR1+ Cells), as indicated at the top, for cells derived from AA1581 (Upper) or AA0857 (Lower), as indicated on the left margin. The cells were stained with either 4A5 (open histograms) or control mAb (filled histograms). The bar indicates the gate used to calculate the proportion of ROR1+ cells, which is indicated in the top right of each histogram.
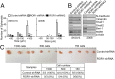
Fig. 5.
Silencing ROR1 inhibits spheroid formation, tumor engraftment, and the expression of Bmi-1 or markers associated with EMT. (A) The average numbers of spheroids assessed in each of three separate wells (±SEM) for SKOV3 (Left) or 2008 (Right) transfected with control-shRNA (open bars), ROR1-shRNA1 (gray bars), or ROR1-shRNA2 (black bars) are depicted in the histograms, as in Fig. 3_E_. (B) Immunoblot analysis of lysates made from SKOV3 or 2008 transfected with control-shRNA, ROR1-shRNA1, or ROR1-shRNA2, as indicated at the top of each lane. The blots were probed for the proteins listed on the right. (C) Representative tumors extirpated from immune-deficient mice engrafted with defined numbers of 2008 cells transfected with either control-shRNA (top row) or ROR1-shRNA (bottom row). (Lower) A table providing the numbers of animals that had tumor at 5 wk post tumor-cell injection. The numbers of injected tumor cells are indicated at the top of the columns, which provide the numbers of mice that developed tumor over the number of mice injected with 2008 cells transfected with control-shRNA or ROR1-shRNA (bottom row). The percentage of injected animals that developed tumor is provided in parentheses.
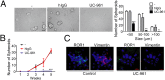
Fig. 6.
ROR1 antibody UC-961 can inhibit spheroid formation of ROR1+ cancer cells. (A, Left) Representative images of spheroids formed by AA0857 ovarian cancer cells cultured for 3 wk in media containing control hIgG or UC-961, each at 50 µg/mL. (Scale bar: 100 µm.) Average numbers of spheroids formed in each of three separate wells with either treatment (±SEM) are indicated in the bar graph (Right), as in Fig. 3_D_. (B) Graph showing the average numbers of detected spheroids over time in three separate wells with either treatment (±SEM). (C) Confocal microscopy of ovarian cancer cell spheroids stained with mAb specific for ROR1 (green) or vimentin (red), after treatment with UC-961 for 48 h. Nuclear staining is in blue. (Scale bar: 10 μm.)
Fig. 7.
ROR1 MAb inhibits tumor engraftment. (A) AA0857 cells (2.5 × 104) were engrafted into Rag2−/−γc−/− mice, which were subsequently treated with control hIgG or UC-961 at times indicated by the arrows. The line graph provides the mean tumor volume over time of control hIgG-treated (red) or UC-961–treated (black) mice ± SEM (n = 3 for each group). Asterisks indicate a significant difference between the mean volume measured in control-treated versus UC-961–treated mice (*P < 0.05, **P < 0.01, ***P < 0.001, using Student’s t test). (B) Representative images of tumors extirpated from mice treated with control hIgG (Upper) or UC-961 (Lower). (C) Average weights of extirpated tumors from mice treated with control hIgG or UC-961 (n = 3). Error bars indicate SEM. (D) Flow-cytometric analysis of cells isolated from tumors extirpated from mice treated with either control hIgG or UC-961 upon staining with ALDOFLOUR ± the ALDH1-inhibitor DEAB, as indicated at the top of each contour plot. The box in the contour plot indicates the gate used to detect cells with ALDH1 activity. The bar graph (Right) provides the average proportion of ALDOFLUOR-staining cells in each tumor population (n = 3) with error bars indicating the SEM. (E) Single-cell suspensions of tumors from AA0857-engrafted immune-deficient mice that were treated with either a control antibody or UC-961 were sorted into ALDH1+ versus ALDH1Neg subgroups by FACS. Defined numbers of ALDH1+ or ALDH1Neg human tumor cells were engrafted into immune-deficient mice, and the development of tumor xenografts was assessed up to 5 mo postengraftment. Frequency of tumorigenic cell and probability estimates were computed using ELDA software.
Similar articles
- Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis.
Cui B, Zhang S, Chen L, Yu J, Widhopf GF 2nd, Fecteau JF, Rassenti LZ, Kipps TJ. Cui B, et al. Cancer Res. 2013 Jun 15;73(12):3649-60. doi: 10.1158/0008-5472.CAN-12-3832. Cancer Res. 2013. PMID: 23771907 Free PMC article. - Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody.
Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ. Zhang S, et al. Proc Natl Acad Sci U S A. 2019 Jan 22;116(4):1370-1377. doi: 10.1073/pnas.1816262116. Epub 2019 Jan 8. Proc Natl Acad Sci U S A. 2019. PMID: 30622177 Free PMC article. - Targeting ROR1 inhibits the self-renewal and invasive ability of glioblastoma stem cells.
Jung EH, Lee HN, Han GY, Kim MJ, Kim CW. Jung EH, et al. Cell Biochem Funct. 2016 Apr;34(3):149-57. doi: 10.1002/cbf.3172. Epub 2016 Feb 28. Cell Biochem Funct. 2016. PMID: 26923195 - Targeting ROR1 identifies new treatment strategies in hematological cancers.
Karvonen H, Niininen W, Murumägi A, Ungureanu D. Karvonen H, et al. Biochem Soc Trans. 2017 Apr 15;45(2):457-464. doi: 10.1042/BST20160272. Biochem Soc Trans. 2017. PMID: 28408486 Review. - Receptor tyrosine kinase-like orphan receptor 1: a novel target for cancer immunotherapy.
Shabani M, Naseri J, Shokri F. Shabani M, et al. Expert Opin Ther Targets. 2015 Jul;19(7):941-55. doi: 10.1517/14728222.2015.1025753. Epub 2015 Apr 2. Expert Opin Ther Targets. 2015. PMID: 25835638 Review.
Cited by
- Anti-Cancer Stem-like Cell Compounds in Clinical Development - An Overview and Critical Appraisal.
Marcucci F, Rumio C, Lefoulon F. Marcucci F, et al. Front Oncol. 2016 May 10;6:115. doi: 10.3389/fonc.2016.00115. eCollection 2016. Front Oncol. 2016. PMID: 27242955 Free PMC article. Review. - Cutting Edge: ROR1/CD19 Receptor Complex Promotes Growth of Mantle Cell Lymphoma Cells Independently of the B Cell Receptor-BTK Signaling Pathway.
Zhang Q, Wang HY, Liu X, Nunez-Cruz S, Jillab M, Melnikov O, Nath K, Glickson J, Wasik MA. Zhang Q, et al. J Immunol. 2019 Oct 15;203(8):2043-2048. doi: 10.4049/jimmunol.1801327. Epub 2019 Sep 18. J Immunol. 2019. PMID: 31534006 Free PMC article. - RON, ROR1 and SUSD2 expression in tissues of endometrial carcinoma patients. Clinicopathological and prognostic implications.
Abdelbary AM, Kaf RM, Lashin ME, Alattar AZ, Elsayed DH, Amin AF, Gertallah LM, Yehia AM. Abdelbary AM, et al. Contemp Oncol (Pozn). 2022;26(2):109-122. doi: 10.5114/wo.2022.118245. Epub 2022 Jun 30. Contemp Oncol (Pozn). 2022. PMID: 35903204 Free PMC article. - IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas.
Huang X, Park H, Greene J, Pao J, Mulvey E, Zhou SX, Albert CM, Moy F, Sachdev D, Yee D, Rader C, Hamby CV, Loeb DM, Cairo MS, Zhou X. Huang X, et al. PLoS One. 2015 Jul 14;10(7):e0133152. doi: 10.1371/journal.pone.0133152. eCollection 2015. PLoS One. 2015. PMID: 26173023 Free PMC article. - Molecular Mechanisms Associated with ROR1-Mediated Drug Resistance: Crosstalk with Hippo-YAP/TAZ and BMI-1 Pathways.
Karvonen H, Barker H, Kaleva L, Niininen W, Ungureanu D. Karvonen H, et al. Cells. 2019 Aug 2;8(8):812. doi: 10.3390/cells8080812. Cells. 2019. PMID: 31382410 Free PMC article. Review.
References
- McGuire WP, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. - PubMed
- Bookman MA, et al. Carboplatin and paclitaxel in ovarian carcinoma: A phase I study of the Gynecologic Oncology Group. J Clin Oncol. 1996;14(6):1895–1902. - PubMed
- Pliarchopoulou K, Pectasides D. Epithelial ovarian cancer: Focus on targeted therapy. Crit Rev Oncol Hematol. 2011;79(1):17–23. - PubMed
- Clarke MF, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous