Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model - PubMed (original) (raw)
. 2015 May;64(5):1728-42.
doi: 10.2337/db14-0758. Epub 2014 Nov 20.
Affiliations
- PMID: 25412623
- PMCID: PMC4407851
- DOI: 10.2337/db14-0758
Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model
Shengbin Huang et al. Diabetes. 2015 May.
Abstract
Diabetes has adverse effects on the brain, especially the hippocampus, which is particularly susceptible to synaptic injury and cognitive dysfunction. The underlying mechanisms and strategies to rescue such injury and dysfunction are not well understood. Using a mouse model of type 2 diabetes (db/db mice) and a human neuronal cell line treated with high concentration of glucose, we demonstrate aberrant mitochondrial morphology, reduced ATP production, and impaired activity of complex I. These mitochondrial abnormalities are induced by imbalanced mitochondrial fusion and fission via a glycogen synthase kinase 3β (GSK3β)/dynamin-related protein-1 (Drp1)-dependent mechanism. Modulation of the Drp1 pathway or inhibition of GSK3β activity restores hippocampal long-term potentiation that is impaired in db/db mice. Our results point to a novel role for mitochondria in diabetes-induced synaptic impairment. Exploration of the mechanisms behind diabetes-induced synaptic deficit may provide a novel treatment for mitochondrial and synaptic injury in patients with diabetes.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
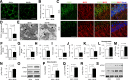
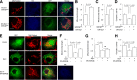
Figure 1
Mitochondrial alterations in hippocampus of db/db mice. A: Representative images of SODII staining of hippocampal neurons from db/m (left) or db/db (right) mice. B: Measurement of mitochondrial density (data presented as the fold increase of the percentage of area occupied by mitochondria in cell body) using MetaMorph software. C: Representative images for COXIV (green) and MAP2 (red) staining in hippocampal pyramidal neurons from db/m and db/db brains. Nuclei were stained by DRAQ5 as shown in blue signals. D: Quantification of immunofluorescence intensity of COXIV in hippocampal pyramidal neurons from db/m and db/db mice. E: Electron microscope images of mitochondria (indicated by arrows) from db/m and db/db hippocampus. Measurements of mitochondrial density (F), length (G), ratio of length to width (H), and form factor (I) from electron microscope images. J and K: Enzymatic activity of complex I (J) and ATP levels (K). L_–_O: Densitometry of immunoreactive bands for Drp1 (L) and Ser616 phosphorylated Drp1 (p-Drp1) (M) that normalized to α-tubulin in hippocampus homogenates of indicated groups and phosphorylated Drp1/Drp1 (N). O: Representative immunoreactive bands for Drp1, phosphorylated Drp1, and α-tubulin. P_–_S: Densitometry of immunoreactive bands for Drp1 (P) and Ser616 phosphorylated Drp1 (Q) relative to HSP60 (mitochondrial marker) in brain mitochondrial fractions isolated from db/m and db/db mice. The level of phosphorylated Drp1/Drp1 was increased in db/db brain mitochondria (R). S: Representative immunoreactive bands for Drp1, phosphorylated Drp1, and HSP60 in mitochondrial fractions in groups of mice as indicated. Data are presented as fold increase relative to db/m mice. Scale bar = 10 or 50 μm in A or C for confocal images, respectively, and 500 nm for electron microscope images in E. N = 6–8 animals per group.
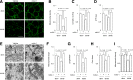
Figure 2
Drp1 activation is responsible for mitochondrial morphology changes in human neuronal SK cells under high-glucose conditions. A and B: The levels of Drp1 in cytosol (A) or in mitochondrial fraction (B) of indicated groups were quantified using ImageJ software. C: Representative images of MitoTracker red (100 nmol/L MitoTracker red was added to cultures 30 min prior to fixation), Drp1 (green), merged images, and an enlarged magnification (as indicated in white rectangle of merge image). Colocalization of Drp1 with MitoTracker red is shown in yellow. The rightmost image is an enlargement of the boxed area as shown in the merged image. D: Representative images for MitoTracker red staining to show mitochondrial morphology in the indicated groups of cells. E_–_G: Measurement of mitochondrial length (E) and density (F) using MetaMorph software and enzymatic activity of complex I (G) with mdivi-1 (+) or vehicle (-) treatment. H: SK cells were transfected with construct encoding GFP vector alone (labeled as vector) or GFP-tagged Drp1K38A (DN-Drp1) and stained with MitoTracker red (100 nmol/L MitoTracker red was added to cultures 30 min prior to fixation). Representative images for GFP (left), MitoTracker red (middle), and the two merged (right) from SK cells transfected with the empty GFP vector (upper panel) or GFP-Drp1K38A (DN-Drp1) (lower panel) construct in the presence of high-glucose concentration (50 mmol/L). I_–_K: Measurement of mitochondrial length (I), density (J), and complex I activity (K) in cells transfected with vector alone (-) or DN-Drp1 (+). L: SK cells were transfected with Drp1 siRNA or control siRNA, and then mitochondrial morphology was examined under confocal microscopy. Representative images for Drp1 immunostaining (left) (green), MitoTracker red (middle), and the two merged (right) from cells treated with control siRNA (upper panel) or Drp1 siRNA (lower panel). M_–_O: Measurement of mitochondrial length (M), density (N), and complex I activity (O) in cells transfected with control siRNA (-) or Drp1 siRNA (+). Data are presented as fold increase relative to control siRNA-treated cells in the presence of high glucose. Scale bar = 10 µm. N ≥ 20 cells per group from two independent experiments. Mdivi-1, 10 μmol/L. H-GLu (-) or (+) denotes culture medium containing 5.5 mmol/L (-) or 50 mmol/L (+).
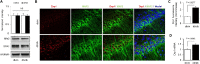
Figure 3
GSK3β/Drp1 interaction in diabetic hippocampus and SK cell line. A: Densitometry of immunoreactive bands. GSK3β (upper right panel) or Drp1 (upper left panel) after immunoprecipitation (IP) of Drp1 or GSK3β, respectively. Lower panels are protein input controls. NI, nonimmune IgG. B: Densitometry of immunoreactive bands for Ser9 phosphorylated GSK3β (p-GSK3β) relative to GSK3β in hippocampus homogenates of the indicated groups. The representative immunoblots for phosphorylated GSK3β or GSK3β are shown in the lower panel. C and D: Quantification of the density of immunoreactive Drp1 and phosphorylated Drp1 bands normalized to α-tubulin (C). The lower panel of C shows representative immunoblots for phosphorylated Drp1, Drp-1, and α-tubulin in hippocampal homogenates from db/m and db/db with (+) or without (-) GSK3β inhibitor TDZD8. The phosphorylated Drp1/Drp1 was calculated (D) from hippocampal slices with or without GSK3β inhibitor TDZD8 (5 μmol/L, treated for 1 h). E_–_G: Densitometry of immunoreactive bands for p-GSK3β (E), Drp1 and phosphorylated Drp1 relative to β-actin (F), and ratio of phosphorylated Drp1 to Drp1 (G) in the SK cells with (+) or without (-) TDZD8 treatment in the presence of high glucose (+) or culture medium (-). H and I: SK cells transfected with dominant negative form of GSK3β (DN-GSK3β) changed phosphorylated Drp1 and Drp1 expression levels (H) and phosphorylated Drp1–to–Drp1 ratio (I). The lower panel of H shows representative immunoblots for the indicated protein in vector (-) or HA-DN-GSK3β–transfected (+) SK cells in the presence of high-glucose levels (+) or vehicle (-). J and K: SK cells transfected with constitutively active mutant of GSK3β (GSK3βS9A) changed phosphorylated Drp1 and Drp1 expression levels relative to β-actin (J) and phosphorylated Drp1–to–Drp1 ratio (K) in indicated groups. The lower panel of J shows representative immunoblots for the indicated protein in vector (-) or GSK3βS9A-transfected (+) SK cells. L: Representative images of triple immunostaining for mitochondria (MitoTracker red), Drp1 (green), and GSK3β (blue) in high glucose (H-Glu)–treated SK cells. The enlarged box area was demonstrated in the lower panel. Arrows pointing to spots denote colocalization of Drp1 with GSK3β and MitoTracker red. Data are presented as fold increase relative to vehicle-treated controls; N = 4–6 animals per group or culture wells from three independent experiments. Scale bar = 10 μm. H-GLu (-) or (+) denotes culture medium containing 5.5 mmol/L (-) or 50 mmol/L (+). (-) indicates the vehicle treatment or control vector transfection and (+) indicates the presence of the indicated drug treatment or indicated construct transfection. IB, immunoblotting.
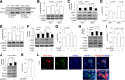
Figure 4
Inactivation of GSK3β prevents high glucose–induced mitochondrial morphology alterations and functional deficit. A_–_D: Representative images of MitoTracker red staining (A) and measurements of mitochondrial length (B), density (C), and complex I activity (D) with (+) or without (-) TDZD8 treatment (5 μmol/L for 1 h) in human SK cells under high-glucose conditions. E_–_H: Representative images of MitoTracker red staining (E) and quantification of mitochondrial length (F), density (G), and complex I activity (H) with empty (-) or HA-DN-GSK3β (+) plasmid transfection in human SK cells under high-glucose conditions. I_–_L: Representative images of MitoTracker staining (I) and quantification of mitochondrial length (J), density (K), and complex I activity (L) with empty (-) or HA-GSK3βS9A (+) plasmid transfection in human SK cells under normal glucose conditions. Data are presented as fold increase relative to vehicle-treated SK cells. N ≥ 20 cells per group from three independent experiments. Scale bar = 10 μm. H-GLu (-) or (+) denotes culture medium containing 5.5 mmol/L (-) or 50 mmol/L (+). (-) Indicates the vehicle treatment or control construct transfection and (+) indicates the presence of the indicated drug treatment or indicated construct transfection.
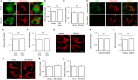
Figure 5
Effect of GSK3β on Drp1-induced mitochondrial morphology and function. A: Representative images showing MitoTracker red staining in cells cotransfected with GFP empty vector (green) and HA-GSK3βS9A (HA-tagged GSK3βS9A [blue]) (upper panel) or with DN-Drp1 (GFP-tagged Drp1K38A [green]) and HA-GSK3βS9A (blue) (bottom panel), respectively. GFP (green) and HA (blue) costaining showed cotransfection with both DN-Drp1 (or GFP empty vector) and GSK3βS9A plasmids (HA-positive staining). B_–_D: Measurement of mitochondrial length (B), density (C), or complex I enzyme activity (D) in the indicated groups of cells. E: Representative images for GFP (green), MitoTracker red, and HA (blue) staining of cells transfected with GFP vector (upper panel), Drp1 (GFP-tagged wild-type Drp1 [middle panel]), or Drp1 (GFP-tagged wild-type Drp1) with DN-GSK3β (HA-tagged) constructs (lower panel). Cells with both GFP and HA-positive staining were cotransfected with both Drp1 and DN-GSK3β plasmids. F_–_H: Measurement of mitochondrial length (F), density (G), or complex I enzyme activity (H) among indicated groups. N ≥ 20 cells per group from two independent experiments. Scale bar = 10 μm. (-) Indicates the vector transfection and (+) indicates the presence of the indicated plasmid transfection.
Figure 6
Effect of Drp1 inhibitor mdivi-1 on mitochondrial changes in diabetic hippocampus. A: Representative images of SODII staining of hippocampal neurons from the indicated groups using confocal microscopy. Scale bar = 10 μm. B_–_D: Quantification of mitochondrial density using MetaMorph software (B), complex I activity (C), and ATP levels (D) in the indicated groups of animals treated with vehicle (-) or mdivi-1 (+). Data are presented as fold increase relative to vehicle-treated db/m mice of indicated hippocampi. E: Representative electron microscope images of mitochondria (indicated by arrows). Scale bar = 500 nm. F_–_H: Quantification of mitochondrial length (F), ratio of length to width (G), form factor (H), and mitochondrial density (I) in the indicated groups of animals treated with vehicle (-) or mdivi-1 (+) from electron microscope images. N = 6–11 animals per group.
Figure 7
Effect of Drp1 inhibitor mdivi-1 or GSK3β inhibition on diabetes-impaired LTP in hippocampal CA1 region. A and B: Basal synaptic transmission (A) and paired-pulse facilitation (PPF) (B) were not significantly affected by diabetes. C: Hippocampal LTP is significantly lower in db/db mice compared with db/m control slices. D_–_F: Hippocampal LTP (D), basal synaptic transmission (E), and paired-pulse facilitation (F) in db/m mouse brain administered with vehicle or mdivi-1 (10 and 25 mg/kg i.p injection daily for 2 weeks) were similar. G: Diabetes-impaired hippocampal LTP was restored by mdivi-1 administration. Upper panel is representative of fEPSP traces indicating the neurotransmission responses before (gray) and after (black) θ-burst stimulation from mdivi-1–treated/nontreated animals. Vertical bar = 1 mV, horizontal bar = 5 ms. H: LTP levels of indicated animal groups were calculated by averaging the last 10 min of fEPSP slope. I_–_K: Blocking GSK3β activation with TDZD8 (5 μmol/L) or LiCl (4 mmol/L) suppressed LTP reduction in db/db hippocampus (I) but did not change the baseline or the LTP levels in db/m controls (J). LTP levels of indicated animal groups were calculated by averaging the last 10 min of fEPSP slope and are illustrated in K. TBS, θ-burst stimulation. Dashed lines in H and K indicate the baseline level. TBS, θ-burst stimulation; V/S, voltage/second. N = 8–14 slices of 5–8 animals per group.
Similar articles
- Blockage of GSK3β-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer's disease.
Yan J, Liu XH, Han MZ, Wang YM, Sun XL, Yu N, Li T, Su B, Chen ZY. Yan J, et al. Neurobiol Aging. 2015 Jan;36(1):211-27. doi: 10.1016/j.neurobiolaging.2014.08.005. Epub 2014 Aug 8. Neurobiol Aging. 2015. PMID: 25192600 - CDK5 phosphorylates DRP1 and drives mitochondrial defects in NMDA-induced neuronal death.
Jahani-Asl A, Huang E, Irrcher I, Rashidian J, Ishihara N, Lagace DC, Slack RS, Park DS. Jahani-Asl A, et al. Hum Mol Genet. 2015 Aug 15;24(16):4573-83. doi: 10.1093/hmg/ddv188. Epub 2015 May 22. Hum Mol Genet. 2015. PMID: 26002103 Free PMC article. - Sevoflurane induced neurotoxicity in neonatal mice links to a GSK3β/Drp1-dependent mitochondrial fission and apoptosis.
Liu J, Li L, Xie P, Zhao X, Shi D, Zhang Y, Pan C, Li T. Liu J, et al. Free Radic Biol Med. 2022 Mar;181:72-81. doi: 10.1016/j.freeradbiomed.2022.01.031. Epub 2022 Feb 2. Free Radic Biol Med. 2022. PMID: 35122996 - [Involvement of Wnt signaling in hippocampal plasticity].
Markevich VA, Salozhin SV, Guliaeva NV. Markevich VA, et al. Ross Fiziol Zh Im I M Sechenova. 2012 Dec;98(12):1460-70. Ross Fiziol Zh Im I M Sechenova. 2012. PMID: 23461192 Review. Russian. - Mitochondrial dynamics in neuronal injury, development and plasticity.
Flippo KH, Strack S. Flippo KH, et al. J Cell Sci. 2017 Feb 15;130(4):671-681. doi: 10.1242/jcs.171017. Epub 2017 Feb 2. J Cell Sci. 2017. PMID: 28154157 Free PMC article. Review.
Cited by
- Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain.
Ruegsegger GN, Vanderboom PM, Dasari S, Klaus KA, Kabiraj P, McCarthy CB, Lucchinetti CF, Nair KS. Ruegsegger GN, et al. JCI Insight. 2019 Sep 19;4(18):e130681. doi: 10.1172/jci.insight.130681. JCI Insight. 2019. PMID: 31534057 Free PMC article. - Sweet Mitochondria: A Shortcut to Alzheimer's Disease.
Moreira PI. Moreira PI. J Alzheimers Dis. 2018;62(3):1391-1401. doi: 10.3233/JAD-170931. J Alzheimers Dis. 2018. PMID: 29562542 Free PMC article. Review. - Caloric restriction improves diabetes-induced cognitive deficits by attenuating neurogranin-associated calcium signaling in high-fat diet-fed mice.
Kim H, Kang H, Heo RW, Jeon BT, Yi CO, Shin HJ, Kim J, Jeong SY, Kwak W, Kim WH, Kang SS, Roh GS. Kim H, et al. J Cereb Blood Flow Metab. 2016 Jun;36(6):1098-110. doi: 10.1177/0271678X15606724. Epub 2015 Oct 5. J Cereb Blood Flow Metab. 2016. PMID: 26661177 Free PMC article. - Dynamics of Dynamin-Related Protein 1 in Alzheimer's Disease and Other Neurodegenerative Diseases.
Oliver D, Reddy PH. Oliver D, et al. Cells. 2019 Aug 23;8(9):961. doi: 10.3390/cells8090961. Cells. 2019. PMID: 31450774 Free PMC article. Review. - Circulating mitochondrial DNA: New indices of type 2 diabetes-related cognitive impairment in Mexican Americans.
Silzer T, Barber R, Sun J, Pathak G, Johnson L, O'Bryant S, Phillips N. Silzer T, et al. PLoS One. 2019 Mar 12;14(3):e0213527. doi: 10.1371/journal.pone.0213527. eCollection 2019. PLoS One. 2019. PMID: 30861027 Free PMC article. Clinical Trial.
References
- Tay SS, Wong WC. Ultrastructural changes in the gracile nucleus of alloxan-induced diabetic rats. Acta Anat (Basel) 1990;139:367–373 - PubMed
- Greenwood CE, Winocur G. High-fat diets, insulin resistance and declining cognitive function. Neurobiol Aging 2005;26(Suppl. 1):42–45 - PubMed
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol 2004;10:36–52 - PubMed
- Biessels GJ, Kamal A, Ramakers GM, et al. . Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes 1996;45:1259–1266 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG037319/AG/NIA NIH HHS/United States
- R01NS065482/NS/NINDS NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- R01 AG044793/AG/NIA NIH HHS/United States
- R01 NS065482/NS/NINDS NIH HHS/United States
- R01AG044793/AG/NIA NIH HHS/United States
- R37AG037319/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous