Strategies for containing Ebola in West Africa - PubMed (original) (raw)
Strategies for containing Ebola in West Africa
Abhishek Pandey et al. Science. 2014.
Abstract
The ongoing Ebola outbreak poses an alarming risk to the countries of West Africa and beyond. To assess the effectiveness of containment strategies, we developed a stochastic model of Ebola transmission between and within the general community, hospitals, and funerals, calibrated to incidence data from Liberia. We find that a combined approach of case isolation, contact-tracing with quarantine, and sanitary funeral practices must be implemented with utmost urgency in order to reverse the growth of the outbreak. As of 19 September, under status quo, our model predicts that the epidemic will continue to spread, generating a predicted 224 (134 to 358) daily cases by 1 December, 280 (184 to 441) by 15 December, and 348 (249 to 545) by 30 December.
Copyright © 2014, American Association for the Advancement of Science.
Figures
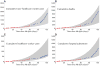
Fig. 1. Model fit to data
(A) Cumulative non–health care worker cases. (B) Cumulative deaths. (C) Cumulative health care worker cases. (D) Cumulative hospital admissions. Cumulative Ebola cases and fatalities were obtained from the Liberian Ministry of Health and Social Welfare Situation Reports nos. 10 to 89 (red circles), to which the model was fit. A 95% prediction interval was generated by 10,000 runs of the model, with parameters randomly sampled from within their confidence intervals (gray fill) (supplementary materials). Validation of the model predictions was provided by comparison with data of the cumulative Ebola cases and fatalities from Situation Reports nos. 89 to 127 (blue circles), representing 12 August 2014 to 19 September 2014, which were not used for model fitting.
Fig. 2. Nonpharmaceutical intervention effectiveness
(A to F) Model predictions of the cumulative number of new cases after 1, 3, and 6 months after 20 September 2014, as well as the probability of fewer than one case daily after these months for (A) 80% reduction in transmission to health care workers combined with different reductions in community transmission, (B) increasing proportions of sanitary burial of hospital deaths, (C) increasing proportions of hospital case isolation, (D) 90% reduction in transmission to health care workers and increasing sanitary burial of hospital deaths, (E) 80% sanitary burial of hospitalized deaths with increasing sanitary burial of community deaths, and (F) 80% case isolation of hospitalized patients with concurrent contact-tracing and quarantine of infected contacts. One thousand simulations of the stochastic model were used to generate the cumulative case count error bars (95% prediction interval) and to estimate the probability of less than one new case per day.
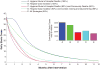
Fig. 3. Effectiveness comparison of individual and combined intervention strategies
Model predictions of the daily number of new and cumulative cases after 6 months for sanitary burial and hospital deaths, sanitary burial of community deaths, case isolation of hospitalized patients, contact-tracing in the community, and quarantine of infected contacts.
Fig. 4. Sensitivities and elasticities with respect to epidemiological parameters
(A to C) Sensitivities and elasticities of cumulative cases under (A) 85% successful sanitary burial of hospital deaths with 95% reduction in transmissibility to health care workers for 6 months (Fig. 2D, blue line); (B) successful sanitary funeral of 90% hospital deaths and 50% community deaths, respectively; and (C) 90% successful hospital case isolation with concurrent contact-tracing and quarantine of 80% infected contacts. We varied the number of pre-outbreak health care workers [SW(0)], the incubation period (1/α), the duration from symptom onset to death if not hospitalized (1/γDG), the duration from symptom onset to recovery if not hospitalized (1/γRG), the duration between symptom onset and hospitalization (1/γH), the hospitalization rate per person for reasons other than Ebola (h), the duration of hospitalization for reasons other than Ebola (1/_f_HG), the number of funeral attendees with close contact to the body (_M_F), the number of hospital visitors per patient (_M_H), the transmission rate at funerals relative to general community (ω), the fraction of asymptomatic infections (1 – ε), and the hospital admission rate (θ). For each parameter varied, we recalibrated and reran the model.
Similar articles
- Evaluating Subcriticality during the Ebola Epidemic in West Africa.
Enanoria WT, Worden L, Liu F, Gao D, Ackley S, Scott J, Deiner M, Mwebaze E, Ip W, Lietman TM, Porco TC. Enanoria WT, et al. PLoS One. 2015 Oct 20;10(10):e0140651. doi: 10.1371/journal.pone.0140651. eCollection 2015. PLoS One. 2015. PMID: 26484544 Free PMC article. - Social Consequences of Ebola Containment Measures in Liberia.
Pellecchia U, Crestani R, Decroo T, Van den Bergh R, Al-Kourdi Y. Pellecchia U, et al. PLoS One. 2015 Dec 9;10(12):e0143036. doi: 10.1371/journal.pone.0143036. eCollection 2015. PLoS One. 2015. PMID: 26650630 Free PMC article. - The Ebola outbreak, 2013-2016: old lessons for new epidemics.
Coltart CE, Lindsey B, Ghinai I, Johnson AM, Heymann DL. Coltart CE, et al. Philos Trans R Soc Lond B Biol Sci. 2017 May 26;372(1721):20160297. doi: 10.1098/rstb.2016.0297. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28396469 Free PMC article. Review. - Potential for broad-scale transmission of Ebola virus disease during the West Africa crisis: lessons for the Global Health security agenda.
Undurraga EA, Carias C, Meltzer MI, Kahn EB. Undurraga EA, et al. Infect Dis Poverty. 2017 Dec 1;6(1):159. doi: 10.1186/s40249-017-0373-4. Infect Dis Poverty. 2017. PMID: 29191243 Free PMC article. - Transmission dynamics and control of Ebola virus disease (EVD): a review.
Chowell G, Nishiura H. Chowell G, et al. BMC Med. 2014 Oct 10;12:196. doi: 10.1186/s12916-014-0196-0. BMC Med. 2014. PMID: 25300956 Free PMC article. Review.
Cited by
- Transmission Dynamics and Final Epidemic Size of Ebola Virus Disease Outbreaks with Varying Interventions.
Barbarossa MV, Dénes A, Kiss G, Nakata Y, Röst G, Vizi Z. Barbarossa MV, et al. PLoS One. 2015 Jul 21;10(7):e0131398. doi: 10.1371/journal.pone.0131398. eCollection 2015. PLoS One. 2015. PMID: 26197242 Free PMC article. - Acute Respiratory Illnesses in Children in the SARS-CoV-2 Pandemic: Prospective Multicenter Study.
Haddadin Z, Schuster JE, Spieker AJ, Rahman H, Blozinski A, Stewart L, Campbell AP, Lively JY, Michaels MG, Williams JV, Boom JA, Sahni LC, Staat M, McNeal M, Selvarangan R, Harrison CJ, Weinberg GA, Szilagyi PG, Englund JA, Klein EJ, Curns AT, Rha B, Langley GE, Hall AJ, Patel MM, Halasa NB. Haddadin Z, et al. Pediatrics. 2021 Aug;148(2):e2021051462. doi: 10.1542/peds.2021-051462. Epub 2021 May 13. Pediatrics. 2021. PMID: 33986150 Free PMC article. - Strong Inference in Mathematical Modeling: A Method for Robust Science in the Twenty-First Century.
Ganusov VV. Ganusov VV. Front Microbiol. 2016 Jul 22;7:1131. doi: 10.3389/fmicb.2016.01131. eCollection 2016. Front Microbiol. 2016. PMID: 27499750 Free PMC article. - Ebola virus disease: from epidemiology to prophylaxis.
Liu WB, Li ZX, Du Y, Cao GW. Liu WB, et al. Mil Med Res. 2015 Mar 10;2:7. doi: 10.1186/s40779-015-0035-4. eCollection 2015. Mil Med Res. 2015. PMID: 26000173 Free PMC article. - Modelling the Role of Human Behaviour in Ebola Virus Disease (EVD) Transmission Dynamics.
Djiomba Njankou SD, Nyabadza F. Djiomba Njankou SD, et al. Comput Math Methods Med. 2022 May 13;2022:4150043. doi: 10.1155/2022/4150043. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35602345 Free PMC article.
References
- World Health Organization. WHO Statement on the Meeting of the International Health Regulations Emergency Committee Regarding the 2014 Ebola Outbreak in West Africa. World Health Organization; 2014. available at www.who.int/mediacentre/news/statements/2014/ebola-20140808/en.
- World Health Organization. WHO: Ebola Response Roadmap Update—15 October 2014. World Health Organization; 2014. pp. 1–11. available at http://apps.who.int/iris/bitstream/10665/136508/1/roadmapsitrep15Oct2014....
- Heymann D, editor. American Public Health Association. Control Commun Dis Man. American Public Health Association; Washington, DC: 2008. pp. 204–206.
- Ksiazek TG, et al. J Infect Dis. 1999;179(suppl. 1):S177–S187. - PubMed
- Khan AS, et al. J Infect Dis. 1999;179(suppl. 1):S76–S86. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 GM087719/GM/NIGMS NIH HHS/United States
- 2 U01 GM087719/GM/NIGMS NIH HHS/United States
- 5 U01 GM105627/GM/NIGMS NIH HHS/United States
- UL1 TR000142/TR/NCATS NIH HHS/United States
- U01 GM105627/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials