Human pDCs preferentially sense enveloped hepatitis A virions - PubMed (original) (raw)
Human pDCs preferentially sense enveloped hepatitis A virions
Zongdi Feng et al. J Clin Invest. 2015 Jan.
Abstract
Unlike other picornaviruses, hepatitis A virus (HAV) is cloaked in host membranes when released from cells, providing protection from neutralizing antibodies and facilitating spread in the liver. Acute HAV infection is typified by minimal type I IFN responses; therefore, we questioned whether plasmacytoid dendritic cells (pDCs), which produce IFN when activated, are capable of sensing enveloped virions (eHAV). Although concentrated nonenveloped virus failed to activate freshly isolated human pDCs, these cells produced substantial amounts of IFN-α via TLR7 signaling when cocultured with infected cells. pDCs required either close contact with infected cells or exposure to concentrated culture supernatants for IFN-α production. In isopycnic and rate-zonal gradients, pDC-activating material cosedimented with eHAV but not membrane-bound acetylcholinesterase, suggesting that eHAV, and not viral RNA exosomes, is responsible for IFN-α induction. pDC activation did not require virus replication and was associated with efficient eHAV uptake, which was facilitated by phosphatidylserine receptors on pDCs. In chimpanzees, pDCs were transiently recruited to the liver early in infection, during or shortly before maximal intrahepatic IFN-stimulated gene expression, but disappeared prior to inflammation onset. Our data reveal that, while membrane envelopment protects HAV against neutralizing antibody, it also facilitates an early but limited detection of HAV infection by pDCs.
Figures
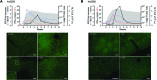
Figure 4. pDCs are transiently recruited to the livers of HAV-infected chimpanzees.
(A) Serum IFN-α and changes in intrahepatic ISG15 and IFIT1 mRNA abundance are shown together with levels of intrahepatic HAV RNA in a chimpanzee, 4x0293, experimentally infected with HAV. The ISG15 response and virologic events have been described previously (12). Sections of archived liver specimens collected from chimpanzee 4x0293 at indicated times prior to and after intravenous challenge with HAV were stained with rabbit polyclonal antibody to human BDCA2. (B) Serum IFN-α and ISG15 and IFIT1 mRNA and ALT activities and intrahepatic HAV RNA in chimpanzee 4x0395 that was similarly infected with HAV (12). Sections of archived liver biopsies from 4x0395 were stained with murine monoclonal antibody 15B to human BDCA2. Scale bars: 200 μm.
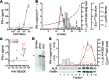
Figure 3. Differential uptake of eHAV versus nonenveloped HAV by pDCs.
(A) pDCs were exposed to equal quantities of gradient-purified eHAV or nonenveloped HAV, and cell-associated viral RNA and supernatant IFN-α levels were determined at intervals. Circles and squares represent cells from 2 individual donors, respectively. (B) pDCs were incubated with eHAV in the presence or absence of annexin V (2 or 10 μg/ml), and cell-associated viral RNA was determined at intervals by RT-qPCR. (C) IFN-α produced by pDCs exposed to concentrated supernatant fluids from mock- or HM175/p16-infected cell cultures in the presence or absence of annexin V (2 μg/ml). (D) IFN-α produced by pDCs exposed to gradient-purified eHAV following inactivation by UV light or cultured with eHAV in the presence of 100 μM HAV 3Cpro inhibitor (3C-inh) (EC90 = 62 μM, CC50 = 3 mM). Efficiency of UV inactivation and 3C inhibitor treatment. Huh-7.5 cells were infected with UV-inactivated eHAV or nontreated eHAV in the presence of 3C-inh, and cell-associated HAV RNA was measured by RT-qPCR and compared with that in cells infected with untreated HAV at 48 hours. (E) pDCs were transfected with HM175/18f genomic RNA in the presence and absence of IRS-661 or subgenomic HAV-luc RNA or a replication incompetent variant HAV-Luc-Δ3D. Supernatant IFN-α levels were measured by ELISA. Results represent the mean ± SEM (n = 2 or 3 cultures) obtained with pDCs from single donors.
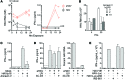
Figure 2. eHAV induces IFN-α production by pDCs.
(A) Serial dilutions of concentrated supernatant (100,000-g pellet) from HAV-infected cells were mixed with pDCs (1 × 106/ml) and incubated for 20 hours. IFN-α levels (mean ± range in replicate assays) are plotted against HAV RNA content. Unconcentrated supernatant from infected (red arrow and triangle) and mock-infected (white square) cells were tested in parallel. (B) Concentrated supernatant fluids from HAV-infected cells were subjected to isopycnic gradient centrifugation, and individual gradient fractions were incubated with pDCs (1 × 106/ml). HAV RNA content of fractions was determined by RT-qPCR. IFN-α production shown represents results from 3 donors (mean ± SEM). AChE activity was measured by enzyme assay. (C) Correlation (Spearman’s test) between eHAV content of isopycnic gradient fractions, shown as genome equivalent per pDC, and IFN-α produced (mean ± range in replicate assays). (D) Northern blot of HAV RNA: full-length in vitro–transcribed HM175/18f HAV RNA, RNA extracted from peak isopycnic gradient fractions containing eHAV, or nonenveloped HAV (mean ± range in replicate assays). (E) Consecutive gradient fractions containing eHAV from a gradient similar to that in B were pooled, concentrated, and subjected to rate-zonal ultracentrifugation. Fractions were collected from the top and assessed for HAV RNA content, AChE activity, and pDC stimulating activity (P105 and P106 indicate individual donors) (mean ± range in replicate assays). Representative results from 1 of 3 independent experiments are shown. Western blots of ALIX and flotillin-1 in the rate-zonal gradient fractions are shown.
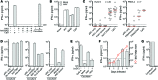
Figure 1. Coculturing with HAV-infected cells induces IFN-α production by pDCs.
(A) pDCs (4 × 105/ml) were exposed to nonenveloped HAV (MOI = 20), either with or without anti-HAV antibody, or cocultured with HM175/p16 virus-infected cells (1 × 106/ml). Supernatant IFN-α was measured at 20 hours by ELISA. CpG DNA (ODN2216, 1 μM) was used as a positive control. LOD, limit of detection. (B) pDCs were exposed to influenza A virus (IAV, 6 HA units/ml), R848 (1 μM), or CpG in the presence or absence of HAV. (C) pDCs from multiple donors were exposed to CpG or HAV or cocultured with mock or HAV-infected Huh-7.5 cells or FRhK-4 cells for 20 hours. Red bars indicate median. (D) pDCs and HAV-infected cells were cocultured in the presence of monoclonal anti-HAV antibody or control IgG, following treatment with either bafilomycin A (BAF, 50 nM) or chloroquine (CLQ, 50 μM) or 1 μM TLR7 antagonist IRS-661 or control IRS. Results are representative of 2 independent experiments. (E) Total or pDC-depleted PBMCs (-pDC) were exposed to 1 μM CpG A or cocultured with mock or HAV-infected Huh-7.5 cells. (F) pDCs were cocultured with cells infected for increasing numbers of days. Supernatant IFN-α (bars) and intracellular HAV RNA (red line) are shown. GE, genome equivalents. (G) pDCs and HAV-infected cells were cocultured with or without separation by a permeable membrane (pore size = 1 μm). Data represent mean ± SEM in A, B, and D–G.
Similar articles
- TIM1 (HAVCR1) Is Not Essential for Cellular Entry of Either Quasi-enveloped or Naked Hepatitis A Virions.
Das A, Hirai-Yuki A, González-López O, Rhein B, Moller-Tank S, Brouillette R, Hensley L, Misumi I, Lovell W, Cullen JM, Whitmire JK, Maury W, Lemon SM. Das A, et al. mBio. 2017 Sep 5;8(5):e00969-17. doi: 10.1128/mBio.00969-17. mBio. 2017. PMID: 28874468 Free PMC article. - Biliary Secretion of Quasi-Enveloped Human Hepatitis A Virus.
Hirai-Yuki A, Hensley L, Whitmire JK, Lemon SM. Hirai-Yuki A, et al. mBio. 2016 Dec 6;7(6):e01998-16. doi: 10.1128/mBio.01998-16. mBio. 2016. PMID: 27923925 Free PMC article. - Characterization of the Plasmacytoid Dendritic Cell Response to Transmitted/Founder and Nontransmitted Variants of HIV-1.
Schwartz JA, Zhang H, Ende Z, Deymier MJ, Lee T, Singer J, Mazzulli T, Hunter E, Ostrowski MA. Schwartz JA, et al. J Virol. 2018 Sep 12;92(19):e00157-18. doi: 10.1128/JVI.00157-18. Print 2018 Oct 1. J Virol. 2018. PMID: 29997203 Free PMC article. - The Dual Role of Exosomes in Hepatitis A and C Virus Transmission and Viral Immune Activation.
Longatti A. Longatti A. Viruses. 2015 Dec 17;7(12):6707-15. doi: 10.3390/v7122967. Viruses. 2015. PMID: 26694453 Free PMC article. Review. - Hepatitis A Virus Genome Organization and Replication Strategy.
McKnight KL, Lemon SM. McKnight KL, et al. Cold Spring Harb Perspect Med. 2018 Dec 3;8(12):a033480. doi: 10.1101/cshperspect.a033480. Cold Spring Harb Perspect Med. 2018. PMID: 29610147 Free PMC article. Review.
Cited by
- A switch from lysosomal degradation to secretory autophagy initiates osteogenic bone metastasis in prostate cancer.
Wei X, Liang M, Deng M, Zheng J, Luo F, Ma Q. Wei X, et al. J Extracell Vesicles. 2024 Nov;13(11):e70002. doi: 10.1002/jev2.70002. J Extracell Vesicles. 2024. PMID: 39497621 Free PMC article. - Identifying and validating MMP family members (MMP2, MMP9, MMP12, and MMP16) as therapeutic targets and biomarkers in kidney renal clear cell carcinoma (KIRC).
Li K, Li D, Hafez B, Bekhit MMS, Jardan YAB, Alanazi FK, Taha EI, Auda SH, Ramzan F, Jamil M. Li K, et al. Oncol Res. 2024 Mar 20;32(4):737-752. doi: 10.32604/or.2023.042925. eCollection 2024. Oncol Res. 2024. PMID: 38560573 Free PMC article. - Trick-or-Trap: Extracellular Vesicles and Viral Transmission.
Bou JV, Taguwa S, Matsuura Y. Bou JV, et al. Vaccines (Basel). 2023 Sep 27;11(10):1532. doi: 10.3390/vaccines11101532. Vaccines (Basel). 2023. PMID: 37896936 Free PMC article. Review. - Identification of useful biomolecular markers in kidney renal clear cell carcinoma: an in silico and in vitro analysis-based study.
Sheikh K, Memon KN, Usman H, Abdel-Maksoud MA, Ullah S, Almanaa TN, Chaudhary A, Jamil M, Gill OBQ, Yar MA, Hussein AM, Zakri AM. Sheikh K, et al. Am J Transl Res. 2023 Sep 15;15(9):5574-5593. eCollection 2023. Am J Transl Res. 2023. PMID: 37854221 Free PMC article. - Characterization and verification of MMP family members as potential biomarkers in kidney clear cell renal carcinoma.
Zhao T, Li X, Li M, Jamil M, Zhang J. Zhao T, et al. Am J Cancer Res. 2023 Sep 15;13(9):3941-3962. eCollection 2023. Am J Cancer Res. 2023. PMID: 37818055 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- C06 RR12087/RR/NCRR NIH HHS/United States
- C06 RR016228/RR/NCRR NIH HHS/United States
- R01 AI103083/AI/NIAID NIH HHS/United States
- U19 AI109965/AI/NIAID NIH HHS/United States
- R01-AI103083/AI/NIAID NIH HHS/United States
- P51 RR13986/RR/NCRR NIH HHS/United States
- U19-AI109965/AI/NIAID NIH HHS/United States
- P51 OD011133/OD/NIH HHS/United States
- C06 RR012087/RR/NCRR NIH HHS/United States
- P51 RR013986/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources