Target-selective protein S-nitrosylation by sequence motif recognition - PubMed (original) (raw)
Target-selective protein S-nitrosylation by sequence motif recognition
Jie Jia et al. Cell. 2014.
Abstract
S-nitrosylation is a ubiquitous protein modification emerging as a principal mechanism of nitric oxide (NO)-mediated signal transduction and cell function. S-nitrosylases can use NO synthase (NOS)-derived NO to modify selected cysteines in target proteins. Despite proteomic identification of over a thousand S-nitrosylated proteins, few S-nitrosylases have been identified. Moreover, mechanisms underlying site-selective S-nitrosylation and the potential role of specific sequence motifs remain largely unknown. Here, we describe a stimulus-inducible, heterotrimeric S-nitrosylase complex consisting of inducible NOS (iNOS), S100A8, and S100A9. S100A9 exhibits transnitrosylase activity, shuttling NO from iNOS to the target protein, whereas S100A8 and S100A9 coordinately direct site selection. A family of proteins S-nitrosylated by iNOS-S100A8/A9 were revealed by proteomic analysis. A conserved I/L-X-C-X2-D/E motif was necessary and sufficient for iNOS-S100A8/A9-mediated S-nitrosylation. These results reveal an elusive parallel between protein S-nitrosylation and phosphorylation, namely, stimulus-dependent posttranslational modification of selected targets by primary sequence motif recognition.
Conflict of interest statement
None of the authors have any financial conflict of interest with the information in this manuscript.
Figures
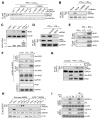
Figure 1. iNOS is Essential for Cys247 _S_-Nitrosylation
(A) L-NMMA suppresses GAPDH Cys247 S-nitrosylation. Human PBM were transfected with HA-GAPDH (WT and Cys-to-Ser mutant). Cells were pre-incubated for 1 hr with L-NMMA (500 μM) and then treated for 24 hr with IFN-γ (500 U/ml) plus LDLox (25 μg/ml). Lysates were subjected to immunoprecipitation (IP) and immunoblot (IB) with antibodies indicated. _S_-nitrosylation was determined by biotin-switch (Biot.-sw). (B) NOS inhibitors block GAPDH S-nitrosylation. PBM were pre-incubated with L-NMMA or 1400W (100 μM), and then with LDLox/IFN-γ. Lysates were subjected to IP and IB or Biot.-sw. analysis. (C) IFN-γ plus LDLox induce iNOS expression in human PBM. Protein and mRNA were determined by IB and qRT-PCR respectively. iNOS expression was normalized to β-actin (mean ± SEM, n = 3 experiments). (D) iNOS is required for inducible GAPDH _S_-nitrosylation. iNOS was depleted by siRNA treatment. Lysates from agonist-treated iNOS-null and control cells were subjected to IB and IP or Biot.-sw. analysis. (E) LDLox/IFN-γ induces iNOS-GAPDH interaction. PBM were incubated for 24 hr with different agonist. Lysates were subjected to IP and IB with indicated antibodies. (F) LDLox induces iNOS-GAPDH interaction. U937 cells were transfected with His-iNOS or empty vector, and then treated with 25 μg/ml native LDL or LDLox. Lysates were subjected to IP and IB with indicated antibodies. (G) iNOS expression is not sufficient for GAPDH _S_-nitrosylation. U937 cells were transfected with His-iNOS. Lysates from control and treated cells were subjected to IP and IB with indicated antibodies or Biot.-sw. analysis. (H) Inducible GAPDH _S_-nitrosylation and L13a degradation are iNOS-dependent. BMDM from wild-type or iNOS−/− mice were treated with IFN-γ, LDLox, or both. Lysates were subjected to IP and IB with indicated antibodies or Biot.-sw. analysis. (I) iNOS contributes to GAIT pathway dysregulation. BMDM from iNOS−/− (top 3 panels) or WT mice (bottom 3 panels) were stimulated for up to 24 hr and then subjected to 35S-Met/Cys metabolic labeling. Lysates were subjected to IP with anti-VEGF-A, -Cp, or -β-actin antibodies, and labeling determined by electrophoresis and autoradiography.
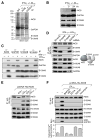
Figure 2. S100A8/A9 Directs iNOS-GAPDH Interaction and GAPDH _S_-Nitrosylation
(A) S100A8 and S100A9 identified as binding partners of iNOS and GAPDH. PBM were incubated for 24 hr with LDLox/IFN-γ. Lysates were subjected to IP with anti-iNOS or anti-GAPDH antibodies, or control IgG, and analyzed by electrophoresis and silver stain. Specific bands were identified by LC-MS analysis. (B) LDLox/IFN-γ does not induce S100A8 or S100A9 expression. Lysates from treated PBM were subjected to IB analysis with antibodies indicated. (C) LDLox/IFN-γ induces assembly of iNOS-S100A8/A9-GAPDH complex. Lysates from treated PBM were subjected to IP and IB analysis with antibodies indicated. (D) S100A9 facilitates binding of S100A8 and GAPDH to iNOS. PBM were transfected with siRNA targeting S100A8, S100A9, or GAPDH. After LDLox/IFN-γ treatment, lysates from treated cells were IP with anti-iNOS antibody and subjected to IB with antibodies indicated (left). Protein interaction model is shown (right). (E) LDLox induces binding of S100A8 and S100A9 to iNOS. PBM were transfected with His-iNOS. After recovery, cells were treated with IFN-γ, LDLox, or both; lysates from treated PBM were subjected to IP and IB analysis with antibodies indicated. (F) LDLox-stimulated intracellular Ca2+ is required for iNOS-S100A8/A9 complex assembly and activity. PBM that ectopically expressed His-iNOS were treated with LDLox in the presence of calcium chelators 15 μM BAPTA-AM or 2 mM EGTA, or with calcium ionophores 5 μM A23187 or 1 μM ionomycin. Lysates were subject to IP and IB with indicated antibodies or Biot.-sw. analysis. Cytosolic calcium was determined with _o_-cresolphthalein at 575 nm, and normalized to total cytosolic protein (mean ± SEM, n = 5 experiments). See also Figure S1.
Figure 3. Transnitrosylase Activity of S100A9 Directs iNOS-S100A8/A9 Complex Assembly and GAPDH _S_-Nitrosylation
(A) S100A9 is essential for inducible GAPDH S-nitrosylation. PBM were transfected with siRNA against S100A8 or S100A9 (or scrambled), and then treated with LDLox/IFN-γ for 24 hr. Lysates were subjected to IP and IB as indicated. GAPDH _S_-nitrosylation was determined by Biot.-sw. (B) LDLox/IFN-γ induces _S_-nitrosylation of S100A9. PBM were pre-incubated with L-NMMA or 1400W, and then with LDLox/IFN-γ. S100A9 _S_-nitrosylation was determined by Biot.-sw. assay. (C) SNO-S100A9 _S_-nitrosylates GAPDH in vitro. Recombinant GAPDH protein was incubated with His-S100A9 pretreated in vitro with 100μM GSH or GSNO, or GSNO-desalted buffer as control. _S_-nitrosylation was determined by Biot.-sw. assay. (D) GAPDH _S_-nitrosylation requires Cys3 of S100A9. PBM were co-transfected with S100A9 3′-UTR-targeting siRNA and recombinant WT myc-S100A9 or C3A mutant. Lysates from treated PBM were subjected to IB with indicated antibodies or Biot.-sw. analysis. (E) S100A9 Cys3 is critical for iNOS-S100A9 interaction. PBM were transfected with recombinant myc-S100A9 (WT or C3A), and then treated with LDLox/IFN-γ. Lysates from treated cells were subjected to IP and IB with indicated antibodies or Biot.-sw. analysis.
Figure 4. S100A8 Determines Site-Selectivity of the iNOS-S100A8/A9 _S_-Nitrosyl- ase Complex
(A) S100A8 directs selective _S_-nitrosylation of GAPDH. PBM were co-transfected with S100A8-specific siRNA and HA-GAPDH (WT or C247S), and then treated with LDLox/IFN-γ for 24 hr. Cell lysates were subjected to IP and IB as shown. Protein _S_-nitrosylation was determined by Biot.-sw. (B) S100A8 depletion leads to inducible GAPDH _S_-nitrosylation on Cys152 and Cys247. PBM were transfected with HA-GAPDH (WT or C247S), or co-transfected with both S100A8 siRNA and HA-GAPDH Cys-to-Ser mutants as indicated. Cells were then treated with LDLox/IFN-γ. Lysates were subjected to IP and IB or Biot.-sw. (C) S100A8 by itself binds GAPDH. Purified His-S100A8 and His-EPRS WHEP-R1 protein (as control) were incubated with GAPDH separately. Protein interaction was determined by IP with anti-His-tag antibody and IB with antibodies as indicated. (D) Fe-BABE determination of S100A8 binding sites on GAPDH. FeBABE-labeled S100A8 bearing cysteine mutations at 10-amino acid intervals in the context of a C42S mutation was incubated with purified N-terminus, HA-GAPDH. Cleavage fragments were determined by IB with anti-HA-tag antibody. Purified recombinant GAPDH and S100A8 proteins were determined by SDS-PAGE and Coomassie blue stain. (E) Structural model of S100A8 binding domain of GAPDH. GAPDH α-helices α1 and α3 (carmine), and Cys247, Cys156, and Cys152 (yellow spheres) are indicated. See also Figure S2.
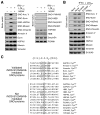
Figure 5. Global _S_-Nitrosylation by the iNOS-S100A8/A9 Complex
(A) iNOS-dependent _S_-nitrosylation of candidates. PBM were pre-incubated with 1400W, and then with LDLox/IFN-γ. _S_-nitrosylated (left) and non-_S_-nitrosylated (right) candidates were determined by Biot.-sw. assay and by IB with antibodies as indicated. (B) S100A9-dependent _S_-nitrosylation of candidates. PBM were transfected with S100A9 (or scrambled) siRNA, and then treated with LDLox/IFN-γ. _S_-nitrosylation of iNOS-dependent candidates was determined by Biot.-sw. Protein expression was determined IB with antibodies indicated. (C) Identification of a putative _S_-nitrosylation consensus sequence in iNOS-S100A8/A9-_S_-nitrosylated proteins. Sequences 10 amino acids upstream and downstream from every cysteine in 5 validated SNO-proteins and 12 sites in 6 proteins found to be not _S_-nitrosylated were aligned by Clustal X. Residues conforming to the consensus motif are boxed.
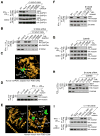
Figure 6. [I/L]-X-C-X2-[D/E] Motif is Necessary and Sufficient for iNOS-S100A8/A9- Directed Protein _S_-Nitrosylation
(A) GAPDH Leu245 and Glu250 are critical for _S_-nitrosylation of Cys247. PBM were transfected with HA-GAPDH (WT and mutant). Cells were then incubated with LDLox/IFN-γ (top 2 panels) or 1 mM GSNO (lower 2 panels). Cell lysates were subjected to IP and IB as indicated; protein _S_-nitrosylation was determined by Biot.-sw. (B) S100A8 directs motif recognition in GAPDH _S_-nitrosylation. PBM were transfected with HA-GAPDH (WT or C247S), or co-transfected with S100A8 siRNA and HA-GAPDH mutant. Cell lysates were subjected to IP and IB with antibodies indicated. Protein _S_-nitrosylation was determined by Biot.-sw. (C) Structural model of _S_-nitrosylation motif in human GAPDH. Leu245 (blue), Cys247 (green), and Glu250 (red) are highlighted. (D) Identification of _S_-nitrosylation site in moesin. PBM were transfected with HA-moesin (WT or Cys-to-Ser mutant). After recovery, cells were treated with LDLox/IFN-γ for 24 hr and _S_-nitrosylation of HA-moesin determined by Biot.-sw. assay. (E) Structural model of human moesin highlighting both cysteine residues and _S_-nitrosylation motif. Left, Localization of moesin Cys residues. Ile115 (blue), Cys117 (green), Glu120 (red) and Cys284 (purple) are highlighted. Right, Model of endogenous and synthetic _S_-nitrosylation motifs in moesin. Ile115 (blue), Cys117 (green) and Glu120 (red) in the conserved motif are highlighted, as are residues mutated in the gain-of-function study, His161 (blue), Leu163 (green) and Asp166 (red). (F) Absence of S100A8 does not alter moesin _S_-nitrosylation site. PBM were transfected with HA-moesin (WT or mutant), or co-transfected with both S100A8 siRNA and mutated HA-moesin. After recovery, cells were treated with LDLox/IFN-γ for 24 hr. Lysates from treated cells were subjected to IP and IB with antibodies indicated; _S_-nitrosylation was determined by Biot.-sw. (G) Ile115 and Glu120 are critical for Cys117 S-nitrosylation of moesin. PBM were transfected with HA-moesin (WT or mutant). After recovery, cells were treated with LDLox/IFN-γ (top 2 panels) or GSNO (lower 2 panels), and _S_-nitrosylation of HA-moesin determined by Biot.-sw. (H) S100A8 recognizes _S_-nitrosylation motif of moesin. PBM were transfected with HA-moesin (WT or C117S), or co-transfected with both S100A8 siRNA and HA-moesin mutant. Lysates from treated cells were subjected to IP and IB as indicated; _S_-nitrosylation was determined by Biot.-sw. (I) Gain-of-function analysis of _S_-nitrosylation motif in moesin. PBM were transfected with HA-moesin (WT or mutant), and then treated with LDLox/IFN-γ. Cell lysates were subjected to IP and IB as indicated. _S_-nitrosylation was determined by Biot.-sw.
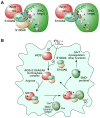
Figure 7. Schematic of iNOS-S100A8/A9-Directed, Site-Selective _S_-Nitrosylation
(A) S100A8 and S100A9 coordinately direct target-selective _S_-nitrosylation by recognition of [I/L]-X-C-X2-[D/E] motif. NO moiety is transferred from iNOS to S100A9 Cys3 and then to cysteine in the target motif (left); or to other accessible cysteine residues in the absence of S100A8 (right). (B) LDLox/IFN-γ induces iNOS expression, iNOS-S100A8/A9 complex assembly, and target-selective S-nitrosylation, which causes GAIT pathway dysregulation, and is likely to influence other myeloid cell functions.
Similar articles
- S-nitrosylated S100A8: novel anti-inflammatory properties.
Lim SY, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, Watts RN, Richardson D, Thomas S, Perry M, Geczy CL. Lim SY, et al. J Immunol. 2008 Oct 15;181(8):5627-36. doi: 10.4049/jimmunol.181.8.5627. J Immunol. 2008. PMID: 18832721 - Inflammation-associated S100 proteins: new mechanisms that regulate function.
Goyette J, Geczy CL. Goyette J, et al. Amino Acids. 2011 Oct;41(4):821-42. doi: 10.1007/s00726-010-0528-0. Epub 2010 Mar 6. Amino Acids. 2011. PMID: 20213444 Review. - Shear flow increases S-nitrosylation of proteins in endothelial cells.
Huang B, Chen SC, Wang DL. Huang B, et al. Cardiovasc Res. 2009 Aug 1;83(3):536-46. doi: 10.1093/cvr/cvp154. Epub 2009 May 15. Cardiovasc Res. 2009. PMID: 19447776 - Protein thiol modification of glyceraldehyde-3-phosphate dehydrogenase as a target for nitric oxide signaling.
Brüne B, Lapetina EG. Brüne B, et al. Genet Eng (N Y). 1995;17:149-64. Genet Eng (N Y). 1995. PMID: 7540026 Review.
Cited by
- Unraveling the Mechanisms of S100A8/A9 in Myocardial Injury and Dysfunction.
Xu Y, Wang Y, Ning K, Bao Y. Xu Y, et al. Curr Issues Mol Biol. 2024 Sep 2;46(9):9707-9720. doi: 10.3390/cimb46090577. Curr Issues Mol Biol. 2024. PMID: 39329929 Free PMC article. Review. - The roles of S-nitrosylation and S-glutathionylation in Alzheimer's disease.
Dyer RR, Ford KI, Robinson RAS. Dyer RR, et al. Methods Enzymol. 2019;626:499-538. doi: 10.1016/bs.mie.2019.08.004. Methods Enzymol. 2019. PMID: 31606089 Free PMC article. Review. - Computational Structural Biology of _S_-nitrosylation of Cancer Targets.
Bignon E, Allega MF, Lucchetta M, Tiberti M, Papaleo E. Bignon E, et al. Front Oncol. 2018 Aug 14;8:272. doi: 10.3389/fonc.2018.00272. eCollection 2018. Front Oncol. 2018. PMID: 30155439 Free PMC article. Review. - Thiol Modifications in the Extracellular Space-Key Proteins in Inflammation and Viral Infection.
Brücksken KA, Loreto Palacio P, Hanschmann EM. Brücksken KA, et al. Front Immunol. 2022 Jun 27;13:932525. doi: 10.3389/fimmu.2022.932525. eCollection 2022. Front Immunol. 2022. PMID: 35833136 Free PMC article. Review. - The enzymatic function of the honorary enzyme: S-nitrosylation of hemoglobin in physiology and medicine.
Premont RT, Singel DJ, Stamler JS. Premont RT, et al. Mol Aspects Med. 2022 Apr;84:101056. doi: 10.1016/j.mam.2021.101056. Epub 2021 Nov 28. Mol Aspects Med. 2022. PMID: 34852941 Free PMC article. Review.
References
- Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, Bornfeldt KE. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation. 2011;123:1216–1226. - PMC - PubMed
- Buzko O, Shokat KM. A kinase sequence database: sequence alignments and family assignment. Bioinformatics. 2002;18:1274–1275. - PubMed
- Chen HT, Hahn S. Binding of TFIIB to RNA polymerase II: Mapping the binding site for the TFIIB zinc ribbon domain within the preinitiation complex. Mol Cell. 2003;12:437–447. - PubMed
- Chen YJ, Ku WC, Lin PY, Chou HC, Khoo KH. S-alkylating labeling strategy for site-specific identification of the s-nitrosoproteome. J Proteome Res. 2010;9:6417–6439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 RR031537/RR/NCRR NIH HHS/United States
- R01 GM086430/GM/NIGMS NIH HHS/United States
- P01 HL076491/HL/NHLBI NIH HHS/United States
- P01 HL029582/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- R01 HL017964/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous