Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia - PubMed (original) (raw)
Comparative Study
Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia
Maria B Rabaglino et al. Hypertension. 2015 Feb.
Erratum in
- Correction.
[No authors listed] [No authors listed] Hypertension. 2015 Jun;65(6):e46-7. doi: 10.1161/HYP.0000000000000027. Hypertension. 2015. PMID: 25972563 No abstract available.
Abstract
Impaired uterine invasion by extravillous trophoblast in early gestation is implicated in the genesis of preeclampsia, a potentially lethal malady of human pregnancy. However, reasons for extravillous trophoblast dysfunction remain unclear because of virtual inaccessibility of early placental and uterine tissues from women who develop preeclampsia, and the absence of animal models in which the disease spontaneously occurs. Consequently, the possibility that deficient or defective maturation of the endometrium (decidualization) may compromise extravillous trophoblast invasion in preeclampsia remains unexplored. Using a bioinformatics approach, we tested this hypothesis identifying 396 differentially expressed genes (DEG) in chorionic villous samples from women at ≈11.5 gestational weeks who developed severe preeclampsia symptoms 6 months later compared with chorionic villous samples from normal pregnancies. A large number, 154 or 40%, overlapped with DEG associated with various stages of normal endometrial maturation before and after implantation as identified by other microarray data sets (P=4.7×10(-14)). One-hundred and sixteen of the 154 DEG or 75% overlapped with DEG associated with normal decidualization in the absence of extravillous trophoblast, ie, late-secretory endometrium (LSE) and endometrium from tubal ectopic pregnancy (EP; P=4.2×10(-9)). Finally, 112 of these 154 DEG or 73% changed in the opposite direction in microarray data sets related to normal endometrial maturation (P=0.01), including 16 DEG upregulated in decidual (relative to peripheral blood) natural killer cells that were downregulated in chorionic villous samples from women who developed preeclampsia (P<0.0001). Taken together, these results suggest that insufficient or defective maturation of endometrium and decidual natural killer cells during the secretory phase and early pregnancy preceded the development of preeclampsia.
Keywords: decidualization; endometrial cycle; natural killer cell; pregnancy; trophoblast.
© 2014 American Heart Association, Inc.
Figures
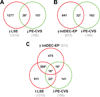
Figure 1. Clusters of genes induced during pre-decidualization and decidualization in ectopic pregnancy are down-regulated in CVS from preeclamptic women
The Venn Diagrams show significant overlap (*P<0.0001 by Pearson’s chi-square test) between DEG down-regulated in CVS from PE women (PE-CVS; relative to CVS from women with normal pregnancy) and DEG up-regulated in: (A) late-secretory endometrium (LSE; relative to proliferative endometrium; 38 DEG, Table S3A) and (B) EP endometrium with intermediate-decidualization (intDEC) changes (relative to EP endometrium without decidualization changes), which lacks extravillous trophoblast (32 DEG, Table S3B). In (C), there is significant overlap (*P<0.0001) between DEG down-regulated in PE-CVS and DEG up-regulated in LSE and EP endometrium with intermediate-decidualized changes (16 DEG, Table S3C).
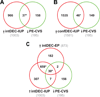
Figure 2. Clusters of genes induced during decidualization in intrauterine and ectopic pregnancy are down-regulated in CVS from preeclamptic women
The Venn Diagrams show significant overlap (*P<0.0001 by Pearson’s chi-square test) between DEG down-regulated in PE-CVS relative to CVS from women with normal pregnancy and DEG up-regulated in: (A) intermediate-decidualized endometrium (intDEC; 37 DEG, Table S4A) and (B) confluent-decidualized endometrium (confDEC; 46 DEG, Table S4B) both from IUP (relative to EP endometrium without decidualization changes) and containing extravillous trophoblast. In (C), there are 32 DEG in common between DEG down-regulated in PE-CVS and DEG up-regulated in EP endometrium with intermediate-decidualized changes and without EVT (also see Figure 1B), and 37 DEG in common between DEG down-regulated in PE-CVS and DEG up-regulated in IUP endometrium with intermediate-decidualized changes (EVT present). The majority of these DEG, in turn, are overlapping (30 DEG, Table S4C; *p<0.0001) suggesting minimal EVT contribution to the overlap.
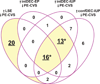
Figure 3. Confluence of gene clusters induced during (pre-) decidualization is down-regulated in CVS from preeclamptic women
There are 20 DEG down-regulated in CVS from PE women (PE-CVS; relative to CVS from women with normal pregnancy) uniquely up-regulated in late-secretory endometrium (LSE, relative to proliferative endometrium). There is also significant overlap (*p<0.0001 by Pearson’s chi-square test) between DEG down-regulated in PE-CVS and DEG up-regulated in EP and IUP endometrium both with intermediate-decidualized (intDEC) changes, and IUP endometrium with confluent-decidualized (confDEC) changes, but not LSE (13 DEG); and in all 4 of the datasets related to different degrees of endometrial maturation (16 DEG). See Table S5 for individual genes and Figure 4 for average expression levels of these genes.
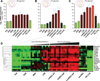
Figure 4. Average gene expression levels (log base 2) in endometrium from different stages of endometrial maturation and CVS from preeclamptic women
(A) Average expression of 20 DEG down-regulated in CVS obtained from women who developed PE (PE-CVS; relative to CVS from women with normal pregnancy) and up-regulated in mid- and late-secretory endometrium (MSE and LSE, respectively; relative to proliferative endometrium, PrE). (B) Average expression of 13 DEG down-regulated in PE-CVS and up-regulated in IUP and EP endometrium with intermediate-decidualized (intDEC) changes, and IUP endometrium with confluent-decidualized (confDEC) changes, but not LSE. (C) Average expression for 16 DEG down-regulated in PE-CVS and upregulated in in all 4 of the datasets related to different degrees of endometrial maturation. (D) Heat map corresponding to Figure 4C. The individual DEG in Figure 4A, B and C are listed in Table S5. EP, ectopic pregnancy; IUP, intrauterine pregnancy; nonDEC, non-decidualized endometrium from EP; ESE, early-secretory endometrium. Significantly different (p<0.05) from: a, PrE; b, ESE; c, MSE; d, LSE; e, intDEC-EP; f, intDEC-IUP; g, nonDEC; h, PE-CVS.
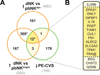
Fig. 5. Clusters of genes induced in decidual Natural Killer cells are down-regulated in CVS from preeclamptic women
(A) The Venn diagram shows significant overlap (*P<0.0001 by Pearson’s chi-square test) between DEG down-regulated in PE-CVS relative to CVS from women with normal pregnancy and DEG up-regulated in decidual Natural Killer cells (dNK) relative to peripheral blood CD56dim or CD56bright NK cells. The official symbols of the overlapping genes are listed in panel (B).
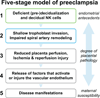
Figure 6. Five-stage model of placental preeclampsia
Based on a bioinformatics approach, the findings of this study raise the possibility that impaired endometrial maturation and deficient decidual NK cell number and/or function in the secretory phase (pre-decidualization) and during early pregnancy (decidualization) precede the development of preeclampsia. As (pre-) decidualization and associated decidual NK cell function are emerging as important players in successful placentation, perturbation of these biological processes may contribute to the etiology of preeclampsia at least in a subset of women who develop the disease. Preeclampsia may arise in some women with little or no endometrial or placental pathology.
Similar articles
- Corin, an enzyme with a putative role in spiral artery remodeling, is up-regulated in late secretory endometrium and first trimester decidua.
Kaitu'u-Lino TJ, Ye L, Tuohey L, Dimitriadis E, Bulmer J, Rogers P, Menkhorst E, Van Sinderen M, Girling JE, Hannan N, Tong S. Kaitu'u-Lino TJ, et al. Hum Reprod. 2013 May;28(5):1172-80. doi: 10.1093/humrep/det028. Epub 2013 Feb 21. Hum Reprod. 2013. PMID: 23434834 - Emerging role for dysregulated decidualization in the genesis of preeclampsia.
Conrad KP, Rabaglino MB, Post Uiterweer ED. Conrad KP, et al. Placenta. 2017 Dec;60:119-129. doi: 10.1016/j.placenta.2017.06.005. Epub 2017 Jun 9. Placenta. 2017. PMID: 28693893 Free PMC article. Review. - The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells.
Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. Quinn KH, et al. J Reprod Immunol. 2011 Sep;91(1-2):76-82. doi: 10.1016/j.jri.2011.05.006. Epub 2011 Jul 23. J Reprod Immunol. 2011. PMID: 21782252 - Evidence for shared molecular pathways of dysregulated decidualization in preeclampsia and endometrial disorders revealed by microarray data integration.
Rabaglino MB, Conrad KP. Rabaglino MB, et al. FASEB J. 2019 Nov;33(11):11682-11695. doi: 10.1096/fj.201900662R. Epub 2019 Aug 7. FASEB J. 2019. PMID: 31356122 Free PMC article. - Decidualization resistance in the origin of preeclampsia.
Garrido-Gómez T, Castillo-Marco N, Cordero T, Simón C. Garrido-Gómez T, et al. Am J Obstet Gynecol. 2022 Feb;226(2S):S886-S894. doi: 10.1016/j.ajog.2020.09.039. Epub 2020 Sep 29. Am J Obstet Gynecol. 2022. PMID: 33007270 Review.
Cited by
- The Roles of Obesity and ASB4 in Preeclampsia Pathogenesis.
Wang Y, Ssengonzi R, Townley-Tilson WHD, Kayashima Y, Maeda-Smithies N, Li F. Wang Y, et al. Int J Mol Sci. 2024 Aug 20;25(16):9017. doi: 10.3390/ijms25169017. Int J Mol Sci. 2024. PMID: 39201703 Free PMC article. Review. - Dysfunction of Decidual Macrophages Is a Potential Risk Factor in the Occurrence of Preeclampsia.
Rong M, Yan X, Zhang H, Zhou C, Zhang C. Rong M, et al. Front Immunol. 2021 May 12;12:655655. doi: 10.3389/fimmu.2021.655655. eCollection 2021. Front Immunol. 2021. PMID: 34054819 Free PMC article. - Investigating Menstruation and Adverse Pregnancy Outcomes: Oxymoron or New Frontier? A Narrative Review.
Tindal K, Cousins FL, Ellery SJ, Palmer KR, Gordon A, Filby CE, Gargett CE, Vollenhoven B, Davies-Tuck ML. Tindal K, et al. J Clin Med. 2024 Jul 29;13(15):4430. doi: 10.3390/jcm13154430. J Clin Med. 2024. PMID: 39124698 Free PMC article. Review. - Cellular immune responses in the pathophysiology of preeclampsia.
Miller D, Motomura K, Galaz J, Gershater M, Lee ED, Romero R, Gomez-Lopez N. Miller D, et al. J Leukoc Biol. 2022 Jan;111(1):237-260. doi: 10.1002/JLB.5RU1120-787RR. Epub 2021 Apr 13. J Leukoc Biol. 2022. PMID: 33847419 Free PMC article. Review. - Establishment and validation of a predictive model of preeclampsia based on transcriptional signatures of 43 genes in decidua basalis and peripheral blood.
Zhang H, Li X, Zhang T, Zhou Q, Zhang C. Zhang H, et al. BMC Bioinformatics. 2022 Dec 7;23(1):527. doi: 10.1186/s12859-022-05086-y. BMC Bioinformatics. 2022. PMID: 36476092 Free PMC article.
References
- Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull. 2003;67:161–176. - PubMed
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. - PubMed
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources