Inside epoxyeicosatrienoic acids and cardiovascular disease - PubMed (original) (raw)
Review
Inside epoxyeicosatrienoic acids and cardiovascular disease
Stefania Tacconelli et al. Front Pharmacol. 2014.
Abstract
Epoxyeicosatrienoic acids (EETs) generated from arachidonic acid through cytochrome P450 (CYP) epoxygenases have many biological functions. Importantly, CYP epoxygenase-derived EETs are involved in the maintenance of cardiovascular homeostasis. In fact, in addition to their potent vasodilating effect, EETs have potent anti-inflammatory properties, inhibit platelet aggregation, promote fibrinolysis, and reduce vascular smooth muscle cell proliferation. All EETs are metabolized to the less active dihydroxyeicosatrienoic acids by soluble epoxide hydrolase (sEH). Numerous evidences support the role of altered EET biosynthesis in the pathophysiology of hypertension and suggest the utility of antihypertensive strategies that increase CYP-derived EET or EET analogs. Indeed, a number of studies have demonstrated that EET analogs and sEH inhibitors induce vasodilation, lower blood pressure and decrease inflammation. Some of these agents are currently under evaluation in clinical trials for treatment of hypertension and diabetes. However, the role of CYP epoxygenases and of the metabolites generated in cancer progression may limit the use of these drugs in humans.
Keywords: cytochrome P450; epoxyeicosatrienoic acids; epoxygenases; hypertension; soluble epoxide hydrolase.
Figures
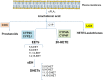
FIGURE 1
The cascade of arachidonic acid (AA). AA is a polyunsaturated omega-6 fatty acid which is released from the sn2 position of membrane phospholipids by the activity of cPLA2. Free AA can be metabolized to eicosanoids through three major pathways: the cyclooxygenase (COX) pathway, the lipoxygenase (LOX) pathway, and the cytochrome P450 (CYP) pathway. In the CYP pathway, AA is converted to epoxyeicosatrienoic acids (EETs) and 20-HETE by CYP epoxygenases and CYP ω-hydroxylases, respectively. All EETs are then further metabolized by soluble epoxide hydrolase (sEH) forming the less active dihydroxyeicosatrienoic acids (DHETs). Modified from Imig and Hammock (2009).
FIGURE 2
Biological effects of EETs.
Similar articles
- Influence of inflammation on cardiovascular protective effects of cytochrome P450 epoxygenase-derived epoxyeicosatrienoic acids.
Shahabi P, Siest G, Visvikis-siest S. Shahabi P, et al. Drug Metab Rev. 2014 Feb;46(1):33-56. doi: 10.3109/03602532.2013.837916. Epub 2013 Sep 16. Drug Metab Rev. 2014. PMID: 24040964 Review. - The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases.
Xu X, Zhang XA, Wang DW. Xu X, et al. Adv Drug Deliv Rev. 2011 Jul 18;63(8):597-609. doi: 10.1016/j.addr.2011.03.006. Epub 2011 Apr 6. Adv Drug Deliv Rev. 2011. PMID: 21477627 Review. - Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation.
Deng Y, Theken KN, Lee CR. Deng Y, et al. J Mol Cell Cardiol. 2010 Feb;48(2):331-41. doi: 10.1016/j.yjmcc.2009.10.022. Epub 2009 Nov 3. J Mol Cell Cardiol. 2010. PMID: 19891972 Free PMC article. Review. - Epoxyeicosatrienoic acid pathway in human health and diseases.
Bellien J, Joannides R. Bellien J, et al. J Cardiovasc Pharmacol. 2013 Mar;61(3):188-96. doi: 10.1097/FJC.0b013e318273b007. J Cardiovasc Pharmacol. 2013. PMID: 23011468 Review. - Arachidonic acid cytochrome P450 epoxygenase pathway.
Spector AA. Spector AA. J Lipid Res. 2009 Apr;50 Suppl(Suppl):S52-6. doi: 10.1194/jlr.R800038-JLR200. Epub 2008 Oct 23. J Lipid Res. 2009. PMID: 18952572 Free PMC article. Review.
Cited by
- Inhibition of Soluble Epoxide Hydrolase 2 Ameliorates Diabetic Keratopathy and Impaired Wound Healing in Mouse Corneas.
Sun H, Lee P, Yan C, Gao N, Wang J, Fan X, Yu FS. Sun H, et al. Diabetes. 2018 Jun;67(6):1162-1172. doi: 10.2337/db17-1336. Epub 2018 Apr 3. Diabetes. 2018. PMID: 29615440 Free PMC article. - Epoxyeicosatrienoic Acids and 20-Hydroxyeicosatetraenoic Acid on Endothelial and Vascular Function.
Imig JD. Imig JD. Adv Pharmacol. 2016;77:105-41. doi: 10.1016/bs.apha.2016.04.003. Epub 2016 May 5. Adv Pharmacol. 2016. PMID: 27451096 Free PMC article. Review. - Cytochrome P450 ω-Hydroxylases in Inflammation and Cancer.
Johnson AL, Edson KZ, Totah RA, Rettie AE. Johnson AL, et al. Adv Pharmacol. 2015;74:223-62. doi: 10.1016/bs.apha.2015.05.002. Epub 2015 Jun 27. Adv Pharmacol. 2015. PMID: 26233909 Free PMC article. Review. - Fluorine and chlorine substituted adamantyl-urea as molecular tools for inhibition of human soluble epoxide hydrolase with picomolar efficacy.
Burmistrov VV, Morisseau C, Danilov DV, Gladkikh BP, D'yachenko VS, Zefirov NA, Zefirova ON, Butov GM, Hammock BD. Burmistrov VV, et al. J Enzyme Inhib Med Chem. 2023 Dec;38(1):2274797. doi: 10.1080/14756366.2023.2274797. Epub 2023 Nov 17. J Enzyme Inhib Med Chem. 2023. PMID: 37975322 Free PMC article. - Gene expression profile during proliferation and differentiation of rainbow trout adipocyte precursor cells.
Bou M, Montfort J, Le Cam A, Rallière C, Lebret V, Gabillard JC, Weil C, Gutiérrez J, Rescan PY, Capilla E, Navarro I. Bou M, et al. BMC Genomics. 2017 May 4;18(1):347. doi: 10.1186/s12864-017-3728-0. BMC Genomics. 2017. PMID: 28472935 Free PMC article.
References
- Anandan S. K., Webb H. K., Chen D., Wang Y. X., Aavula B. R., Cases S., et al. (2011). 1-(1-acetyl-piperidin-4-yl)-3-adamantan-1-yl-urea. (AR9281) as a potent, selective, and orally available soluble epoxide hydrolase inhibitor with efficacy in rodent models of hypertension and dysglycemia. Bioorg. Med. Chem. Lett. 21 983–988 10.1016/j.bmcl.2010.12.042 - DOI - PMC - PubMed
- Archer S. L., Gragasin F. S., Wu X., Wang S., McMurtry S., Kim D. H., et al. (2003). Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12. (–)epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation 107 769–776 10.1161/01.CIR.0000047278.28407.C2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources