Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients - PubMed (original) (raw)
Clinical Trial
. 2014 Nov 27;515(7528):563-7.
doi: 10.1038/nature14011.
Jean-Charles Soria 2, Marcin Kowanetz 3, Gregg D Fine 3, Omid Hamid 4, Michael S Gordon 5, Jeffery A Sosman 6, David F McDermott 7, John D Powderly 8, Scott N Gettinger 1, Holbrook E K Kohrt 9, Leora Horn 10, Donald P Lawrence 11, Sandra Rost 3, Maya Leabman 3, Yuanyuan Xiao 3, Ahmad Mokatrin 3, Hartmut Koeppen 3, Priti S Hegde 3, Ira Mellman 3, Daniel S Chen 3, F Stephen Hodi 12
Affiliations
- PMID: 25428504
- PMCID: PMC4836193
- DOI: 10.1038/nature14011
Clinical Trial
Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients
Roy S Herbst et al. Nature. 2014.
Abstract
The development of human cancer is a multistep process characterized by the accumulation of genetic and epigenetic alterations that drive or reflect tumour progression. These changes distinguish cancer cells from their normal counterparts, allowing tumours to be recognized as foreign by the immune system. However, tumours are rarely rejected spontaneously, reflecting their ability to maintain an immunosuppressive microenvironment. Programmed death-ligand 1 (PD-L1; also called B7-H1 or CD274), which is expressed on many cancer and immune cells, plays an important part in blocking the 'cancer immunity cycle' by binding programmed death-1 (PD-1) and B7.1 (CD80), both of which are negative regulators of T-lymphocyte activation. Binding of PD-L1 to its receptors suppresses T-cell migration, proliferation and secretion of cytotoxic mediators, and restricts tumour cell killing. The PD-L1-PD-1 axis protects the host from overactive T-effector cells not only in cancer but also during microbial infections. Blocking PD-L1 should therefore enhance anticancer immunity, but little is known about predictive factors of efficacy. This study was designed to evaluate the safety, activity and biomarkers of PD-L1 inhibition using the engineered humanized antibody MPDL3280A. Here we show that across multiple cancer types, responses (as evaluated by Response Evaluation Criteria in Solid Tumours, version 1.1) were observed in patients with tumours expressing high levels of PD-L1, especially when PD-L1 was expressed by tumour-infiltrating immune cells. Furthermore, responses were associated with T-helper type 1 (TH1) gene expression, CTLA4 expression and the absence of fractalkine (CX3CL1) in baseline tumour specimens. Together, these data suggest that MPDL3280A is most effective in patients in which pre-existing immunity is suppressed by PD-L1, and is re-invigorated on antibody treatment.
Trial registration: ClinicalTrials.gov NCT01375842.
Figures
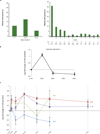
Extended Data Figure 1. Study design and pharmacokinetics
a, Summary of PCD4989g design, including screening, treatment period and follow-up. b, Summary of the dose-escalation (patient numbers are given in the lower right corners) and dose-expansion cohorts. c, Pharmacokinetics for MPDL3280A. Error bars indicate standard deviation. C, cycle; CR, complete response; CT, computed tomography; DLT, dose-limiting toxicity; IV, intravenous; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; RCC, renal cell carcinoma; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
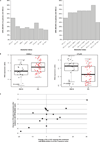
Extended Data Figure 2. Pyrexia and biomarkers over time
a, The graph on the left shows patients who developed pyrexia during the first cycle of MPDL3280A treatment by day. The graph on the right shows patients who developed pyrexia during all cycles of treatment with MPDL3280A. The percentage of patients with pyrexia and the number of patients available for analysis at each time point is indicated below the graph. b, Changes in CD81HLA-DR1Ki-671 cells over the first 5 cycles of treatment with MPDL3280A. The y axis represents the log2 fold-change versus C1D1 pre-dose level. Error bars are standard error of the mean. Samples from 164 patients were examined at cycle (C) 1 day (D) 1 (C1D1) and 145 patients at C2D1. The P value for the difference in fold change between C2D1 versus C1D1 was, 0.00001. c, Changes in IFN-c, ITAC, IL-18 and IL-6 levels shown over the first seven 21-day cycles of treatment with MPDL3280A. To measure fold changes in IFN-c and IL-6 levels, 112 and 109 patient samples were examined for C1D1 and C2D1, respectively. To measure fold changes in IL-18, 260 and 253 patient samples were examined for C1D1 and C2D1, respectively; to measure ITAC, 262 and 256 patient samples were examined for C1D1 and C2D1, respectively. The adjusted P values comparing C2D1 versus C1D1 were 0.94 for IFN-c, 1 for IL-6, 0.00001 for IL-18 and, 0.00001 for ITAC. Error bars are standard error of the mean.
Extended Data Figure 3. Antitumour activity of MPDL3280A in patients with all tumour types
a, Time to response and the duration of study treatment by tumour type and IHC (tumour-infiltrating immune cell) status. b, Representative images (103 magnification) of PD-L1 and CD8 immunohistochemistry (IHC) staining from a pre-treatment tumour biopsy sample from a patient with NSCLC. The patient’s best response to MPDL3280A was a partial response. CRC, colorectal cancer; IC, tumour-infiltrating immune cells; ND, not determined; NSCLC, non-small cell lung cancer; PD, progressive disease; RCC, renal cell carcinoma; TC, tumour cells.
Extended Data Figure 4. Antitumour activity of MPDL3280A by PD-L1 immunohistochemistry (IHC) status
a, The overall objective response rate (ORR; best response of complete response (CR) and partial response (PR)), stable disease (SD) as the best response rate and progressive disease (PD) as the best response rate for patients with non-small cell lung cancer (NSCLC) who received MPDL3280A by PD-L1 IHC (tumour-infiltrating immune cell (IC)) status. Overall, 53 patients with NSCLC were evaluated: 6 patients had an IHC (IC) score of 3; 7 patients had a score of 2; 13 patients had a score of 1; and 20 patients had a score of 0. Seven patients had an unknown IHC status (data not shown). Patients with no post-first dose assessment were not estimable and not plotted (1 in IHC 1 and 1 in IHC 2), but were included in the denominator for purposes of calculating ORR. Using a logistic regression model, PD-L1 by IHC (IC) was significantly associated with response to MPDL3280A (P = 0.015). b, The ORR, SD as best response rate, and PD as best response rate for patients with all tumour types who received MPDL3280A by PD-L1 IHC (IC) status. Patients with no post-first dose assessment were not estimable (NE) and not plotted (1 in IHC 0, 2 in IHC 1, 1 in IHC 2 and 1 in IHC 3), but were included in the denominator for purposes of calculating ORR. Using a logistic regression model, PD-L1 by IHC (IC) was significantly associated with response to MPDL3280A (P = 0.007). c, The ORR, SD as best response rate, and PD as best response rate for patients with NSCLC who received MPDL3280A by PD-L1 IHC (TC) status. Overall, 53 patients with NSCLC were evaluated: 8 patients had an IHC score of 3; 1 patient had a score of 2; 3 patients had a score of 1; and 34 patients had a score of 0. Seven patients had an unknown IHC status (data not shown). Patients with no post-first dose assessment were not estimable and not plotted (2 in IHC 0), but were included in the denominator for purposes of calculating ORR. All responses were confirmed except for in 1 patient. Using a logistic regression model, PD-L1 by IHC (TC) did not meet statistical significance for association with response (P = 0.920). d, The ORR and SD and PD best response rates for patients with all tumour types who received MPDL3280A by PD-L1 IHC (TC) status. Overall, 175 patients with all tumour types were evaluated: 15 patients had an IHC score of 3; 3 patients had a score of 2; 11 patients had a score of 1; and 121 patients had a score of 0; 25 patients had an unknown IHC status (data not shown). Patients with no post-first dose assessment were not estimable and not plotted (3 in IHC 0, 1 in IHC 1 and 1 in IHC 3), but were included in the denominator for purposes of calculating ORR. All responses were confirmed except for in 1 patient with NSCLC, 1 patient with RCC and 2 patients with melanoma. Using a logistic regression model, PD-L1 by IHC (TC) did not meet statistical significance for the association with response (P = 0.079).
Extended Data Figure 5. Biomarkers and antitumour activity of MPDL3280A
a, Objective response rates (ORRs) were plotted by the biomarker status of tumour samples from patients who had tumour available for both immunohistochemistry (IHC) staining and immunochip (n = 37). Left: ORRs for patient sub-populations defined by positivity in a single biomarker as indicated. Right: ORRs for patients positive for PD-L1 and one other marker as indicated. PD-L1 positivity was defined as 55% of tumour-infiltrating immune cells (ICs) staining for PD-L1 by IHC. For PD-L2, IDO1, LAG3, TIM3, CTLA4, B7-H3 and B7-H4 positivity was determined by gene expression 5 the median. b, Baseline CX3CL1 and CTLA4 gene expression levels are binned according to patient response to treatment with MPDL3280A. Includes patients with all tumour types. P values were determined by _t_-test. c, Changes in PD-L1 (IHC) versus interferon (IFN)-c (qPCR) expression after treatment with MPDL3280A in patients with paired serial biopsies. Pearson correlation coefficient = 0.70. CR, complete response; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours.
Extended Data Figure 6. Gene expression levels according to patient response and tumour type
Baseline _IFN_c, IDO1 and CXCL9 gene expression levels are binned according to patient response to treatment with MPDL3280A. Patients are grouped according to tumour type. P values were determined by _t_-test. CR, complete response; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; RCC, renal cell carcinoma.
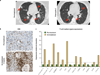
Extended Data Figure 7. Biomarker analyses for a responding patient receiving MPDL3280A
A patient with PD-L1-positive (IHC (IC) 3) renal cell carcinoma who responded to treatment with MPDL3280A. a, Representative computed tomography scans taken at pre-treatment and at post-cycle. Red arrows indicate the location of tumours or where tumours used to be. b, Top panel: representative image of CD8 IHC staining from a pre-treatment tumour biopsy (403 magnification). Bottom panel: representative image of CD8 IHC staining from a tumour biopsy of a shrinking lesion during week 4 of treatment with MPDL3280A that demonstrates an increase in CD81 T-cell infiltration (203 magnification). c, Gene-expression analysis of T-cell markers pre-treatment (set to 1) and on treatment at week 4. Data were normalized to the baseline. Twofold was the cutoff for a gene to be considered induced (indicated by the dashed line).
Extended Data Figure 8. Biomarker analyses of patients with immunological ignorance and a non-functional immune response
a, A patient with PD-L1-negative (IHC (IC) 0) breast cancer whose best response to MPDL3280A was progressive disease with immunological ignorance. Top: representative image of PD-L1 IHC staining from a pre-treatment tumour biopsy. Bottom: representative image of PD-L1 IHC staining from a tumour biopsy during week 9 of treatment with MPDL3280A. Both images are at 103 magnification. b, A patient with PD-L1 IHC 1 melanoma whose best response to MPDL3280A was progressive disease with a non-functional immune response. Gene-expression analysis of T-cell markers at pre-treatment (set to 1) and on treatment at week 6. Data were normalized to baseline. Twofold was the cutoff for a gene to be considered induced (indicated by the dashed line).
Extended Data Figure 9. Biomarker analyses of a patient with an excluded infiltrate
A patient with PD-L1-negative (IHC (IC) 1) melanoma whose best response to MPDL3280A was progressive disease with an ‘excluded infiltrate’. Gene expression analysis of T-cell markers at pre-treatment (set to 1) and on treatment at week 6. Data were normalized to baseline. Twofold was the cutoff for a gene to be considered induced (indicated by the dashed line).
Extended Data Figure 10. Characterization of MPDL3280A
a, Affinity measurements conducted by surface plasmon resonance and binding to PD-L1-expressing human cells using MPDL3280A and trastuzumab. Data from a representative experiment (1 of 4) is shown here. b, In vitro assays for antibody-dependent cellular cytotoxicity. The wild-type antibody is unmodified and the Fc modified MPDL3280A antibody has an engineered Fc domain. GPE, glycophorin E; MFI, mean of fluorescence intensity.
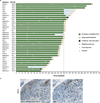
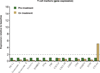
Figure 1. Programmed death-ligand 1 (PD-L1) prevalence and expression
a, PD-L1 prevalence by immunohistochemistry (IHC) in samples collected for PCD4989g. PD-L1 positivity was defined as $5% of tumour-infiltrating immune cells (ICs) or tumour cells (TCs) staining for PD-L1 by IHC. b, Representative images of PD-L1 by IHC (brown) in tumours from patients with non-small cell lung cancer (NSCLC). The PD-L1-negative image is at 203 magnification, other images at 403 magnification. c, Co-localization of PD-L1 with selected tumour-infiltrating immune cell and tumour cell markers by immunofluorescence in NSCLC and melanoma tumours. PD-L1 staining in red; markers of tumour-infiltrating immune cells and tumour cells in green; and DAPI staining in blue. Areas of overlap are indicated with white arrowheads. All four images are at 403 resolution. Markers of tumour-infiltrating immune cells: CD163 (macrophages), CD11c (dendritic cells) and CD3 (T cells). Marker of tumour cells: cytokeratin (CK). CRC, colorectal cancer; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma.
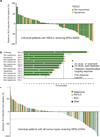
Figure 2. Antitumour activity of MPDL3280A
a, A waterfall plot of patients with non-small cell lung cancer (NSCLC) measuring the maximum reduction from baseline in the sum of the longest diameter (SLD) for target lesions; 120% and 230% are marked by dashed lines. b, The time to response (Response Evaluation Criteria in Solid Tumours version 1.1) and the duration of study treatment for patients with NSCLC. The patient with progressive disease (PD) experienced ongoing clinical benefit as judged by the investigator. All but one response was confirmed. c, A waterfall plot of patients with all tumour types measuring the maximum reduction from baseline in the SLD for target lesions; 120% and 230% are marked by dashed lines. IC, tumour-infiltrating immune cells; IHC, immunohistochemistry; RCC, renal cell carcinoma.
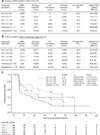
Figure 3. Antitumour activity of MPDL3280A by immunohistochemistry (IHC) tumour-infiltrating immune cell (IC) and biomarker status
a, Table of antitumour activity in patients with NSCLC by PD-L1 IHC (IC) status. Patients with no post-first dose assessment were not estimable (NE; 1 with IHC 1 and 1 with IHC 2), but were included in the denominator for calculating objective response rate (ORR). b, Table of antitumour activity in patients with all tumour types by PD-L1 IHC (IC) status. Patients with no post-first dose assessment were not estimable and not included in the table (1 with IHC 0, 2 with IHC 1, 1 with IHC 2 and 1 with IHC 3), but were included in the denominator for calculating ORR. c, Kaplan–Meier curve showing the phase I percentage of progression-free survival by patient IHC (IC) status. Censored data are indicated by vertical tick marks. CI, confidence interval; NSCLC, non-small cell lung cancer; ORR, objective response rate; PD, progressive disease; PFS, progression free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
Figure 4. Biomarker status and response to MPDL3280A
a, Table summarizing the frequency of patients with an increase in PD-L1-ositive tumour-infiltrating immune cells (ICs) and tumour cells (TCs) by change in the sum of the longest diameter (SLD) and by response to MPDL3280A in patients with paired serial biopsies. There were 28 paired serial biopsies. Of these, 16 tumours were melanoma, 4 were renal cell carcinoma, 4 were non-small cell lung cancer, 2 were head and neck carcinoma, and 2 were colorectal cancer. Patients with an increase of $5% in PDL1-expressing tumour cells and tumour-infiltrating immune cells were identified as having increased PD-L1 expression by IHC after treatment with MPDL3280A. The patient who was unevaluable for SLD had the responding tumour excised for biomarker analysis. This table also includes one patient with progressive disease (PD) by RECIST version 1.1 but without post-dose SLD measures. b, Left: ‘immunological ignorance’ visualized by CD8 IHC. See Extended Data Fig. 8a for additional information. Middle: ‘non-functional immune response’ visualized by CD8 IHC. See Extended Data Fig. 8b for additional information. Right: ‘excluded infiltrate’ visualized by CD8 IHC. See Extended Data Fig. 9 for additional information. All images are at 103 magnification. PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
Similar articles
- MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. Powles T, et al. Nature. 2014 Nov 27;515(7528):558-62. doi: 10.1038/nature13904. Nature. 2014. PMID: 25428503 Clinical Trial. - A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study.
Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, Hollebecque A, Scoazec JY, Marabelle A, Massard C, Soria JC, Robert C, Paragios N, Deutsch E, Ferté C. Sun R, et al. Lancet Oncol. 2018 Sep;19(9):1180-1191. doi: 10.1016/S1470-2045(18)30413-3. Epub 2018 Aug 14. Lancet Oncol. 2018. PMID: 30120041 - Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors.
De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A, Laghouati S, Robert C, Marabelle A, Guettier C, Samuel D. De Martin E, et al. J Hepatol. 2018 Jun;68(6):1181-1190. doi: 10.1016/j.jhep.2018.01.033. Epub 2018 Feb 8. J Hepatol. 2018. PMID: 29427729 - The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.
Mahoney KM, Freeman GJ, McDermott DF. Mahoney KM, et al. Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review. - Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer.
Kurozumi S, Fujii T, Matsumoto H, Inoue K, Kurosumi M, Horiguchi J, Kuwano H. Kurozumi S, et al. Med Mol Morphol. 2017 Dec;50(4):185-194. doi: 10.1007/s00795-017-0170-y. Epub 2017 Sep 21. Med Mol Morphol. 2017. PMID: 28936553 Review.
Cited by
- DDR1 is identified as an immunotherapy target for microsatellite stable colon cancer by CRISPR screening.
Wu M, Ma W, Lv G, Wang X, Li C, Chen X, Peng X, Tang C, Pan Z, Liu R, Chen G, Zhang R. Wu M, et al. NPJ Precis Oncol. 2024 Nov 7;8(1):253. doi: 10.1038/s41698-024-00743-2. NPJ Precis Oncol. 2024. PMID: 39511298 - Integrated temporal transcriptional and epigenetic single-cell analysis reveals the intrarenal immune characteristics in an early-stage model of IgA nephropathy during its acute injury.
Xu C, Zhang Y, Zhou J, Zhang J, Dong H, Chen X, Tian Y, Wu Y. Xu C, et al. Front Immunol. 2024 Oct 18;15:1405748. doi: 10.3389/fimmu.2024.1405748. eCollection 2024. Front Immunol. 2024. PMID: 39493754 Free PMC article. - FOLFOX-Based Hepatic Arterial Infusion Chemotherapy with Sequential Drug-Eluting Bead Transarterial Chemoembolization for Unresectable Large Hepatocellular Carcinoma: A Single-Center Retrospective Cohort Study.
Zhao R, Zhou J, Zheng Z, Xiong X, Wang Q, Li S, Wei W, Guo R. Zhao R, et al. J Hepatocell Carcinoma. 2024 Oct 28;11:2087-2099. doi: 10.2147/JHC.S493577. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 39493266 Free PMC article. - Biomarkers to predict the benefits of immune‑checkpoint blockade‑based therapy in patients with malignant peritoneal mesothelioma (Review).
Wang C, Zhao Y, Liang W. Wang C, et al. Oncol Lett. 2024 Oct 9;28(6):600. doi: 10.3892/ol.2024.14733. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39483967 Free PMC article. Review.
References
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012;18:6580–6587. - PubMed
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous