Components and regulation of nuclear transport processes - PubMed (original) (raw)
Review
. 2015 Feb;282(3):445-62.
doi: 10.1111/febs.13163. Epub 2014 Dec 22.
Affiliations
- PMID: 25429850
- PMCID: PMC7163960
- DOI: 10.1111/febs.13163
Review
Components and regulation of nuclear transport processes
Bastien Cautain et al. FEBS J. 2015 Feb.
Abstract
The spatial separation of DNA replication and gene transcription in the nucleus and protein translation in the cytoplasm is a uniform principle of eukaryotic cells. This compartmentalization imposes a requirement for a transport network of macromolecules to shuttle these components in and out of the nucleus. This nucleo-cytoplasmic transport of macromolecules is critical for both cell physiology and pathology. Consequently, investigating its regulation and disease-associated alterations can reveal novel therapeutic approaches to fight human diseases, such as cancer or viral infection. The characterization of the nuclear pore complex, the identification of transport signals and transport receptors, as well as the characterization of the Ran system (providing the energy source for efficient cargo transport) has greatly facilitated our understanding of the components, mechanisms and regulation of the nucleo-cytoplasmic transport of proteins in our cells. Here we review this knowledge with a specific emphasis on the selection of disease-relevant molecular targets for potential therapeutic intervention.
Keywords: anti-cancer therapy; anti-viral therapy; karyopherins; nuclear export; nuclear import; nuclear pore complex; nuclear trafficking.
© 2014 FEBS.
Figures
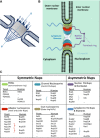
Figure 1
Overall structure and molecular composition of the nuclear pore complexes (
NPC
s). (A) General structural features of the
NPC
. (B) A schematic model of the
NPC
. In this model, the
NPC
is divided into several groups according to their location and structural characteristics. The symmetrical core is composed of membrane‐anchored
POMS
(transmembrane ring), channel Nups (central
FG
‐Nups) and scaffold Nups composed by adaptor Nups (inner and linker Nups) and coat Nups (outer ring). Asymmetric parts of the pore are the nuclear
FG
‐Nups and the basket plus the cytoplasmic
FG
‐Nups and filaments. (C) The yeast and vertebrate homolog Nups that are known to constitute each
NPC
substructure are listed. Symmetric Nups are equally distributed on the cytoplasmic and nucleoplasmic parts of the
NPC
and form the core region. Asymmetric Nups form the nuclear basket and the cytoplasmic filaments. They serve as docking sites for transport factors and include associated m
RNA
export factors. See the main text for more information.
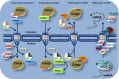
Figure 2
Schematic overview of Ran‐dependent nucleo‐cytoplasmic transport. Nuclear export.
CRM
1 exports a great part of
NES
‐containing protein. Nuclear import. Importin‐α (Imp‐α)/importin‐β (Imp‐β) heterodimer (designated as α and β) and karyopherin‐β2 mediate the import of
NLS
‐containing proteins. See the main text for details.
Similar articles
- Nucleocytoplasmic transport of proteins.
Sorokin AV, Kim ER, Ovchinnikov LP. Sorokin AV, et al. Biochemistry (Mosc). 2007 Dec;72(13):1439-57. doi: 10.1134/s0006297907130032. Biochemistry (Mosc). 2007. PMID: 18282135 Review. - Protein export from the nucleus.
Ossareh-Nazari B, Gwizdek C, Dargemont C. Ossareh-Nazari B, et al. Traffic. 2001 Oct;2(10):684-9. doi: 10.1034/j.1600-0854.2001.21002.x. Traffic. 2001. PMID: 11576444 Review. - The nucleoporin Nup358/RanBP2 promotes nuclear import in a cargo- and transport receptor-specific manner.
Wälde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U, Wiemann S, Kehlenbach RH. Wälde S, et al. Traffic. 2012 Feb;13(2):218-33. doi: 10.1111/j.1600-0854.2011.01302.x. Epub 2011 Nov 21. Traffic. 2012. PMID: 21995724 - Mechanisms of receptor-mediated nuclear import and nuclear export.
Pemberton LF, Paschal BM. Pemberton LF, et al. Traffic. 2005 Mar;6(3):187-98. doi: 10.1111/j.1600-0854.2005.00270.x. Traffic. 2005. PMID: 15702987 Review. - Nuclear transport mechanisms.
Quimby BB, Corbett AH. Quimby BB, et al. Cell Mol Life Sci. 2001 Nov;58(12-13):1766-73. doi: 10.1007/PL00000816. Cell Mol Life Sci. 2001. PMID: 11766877 Free PMC article. Review.
Cited by
- Loss of Lamin A leads to the nuclear translocation of AGO2 and compromised RNA interference.
Lobo V, Nowak I, Fernandez C, Correa Muler AI, Westholm JO, Huang HC, Fabrik I, Huynh HT, Shcherbinina E, Poyraz M, Härtlova A, Benhalevy D, Angeletti D, Sarshad AA. Lobo V, et al. Nucleic Acids Res. 2024 Sep 9;52(16):9917-9935. doi: 10.1093/nar/gkae589. Nucleic Acids Res. 2024. PMID: 38994560 Free PMC article. - Impact of Nucleoporin-Mediated Chromatin Localization and Nuclear Architecture on HIV Integration Site Selection.
Wong RW, Mamede JI, Hope TJ. Wong RW, et al. J Virol. 2015 Oct;89(19):9702-5. doi: 10.1128/JVI.01669-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136574 Free PMC article. Review. - RCC1 regulation of subcellular protein localization via Ran GTPase drives pancreatic ductal adenocarcinoma growth.
Bannoura SF, Aboukameel A, Khan HY, Uddin MH, Jang H, Beal EW, Thangasamy A, Shi Y, Kim S, Wagner KU, Beydoun R, El-Rayes BF, Philip PA, Mohammad RM, Saif MW, Al-Hallak MN, Pasche BC, Azmi AS. Bannoura SF, et al. Cancer Lett. 2024 Nov 1;604:217275. doi: 10.1016/j.canlet.2024.217275. Epub 2024 Sep 24. Cancer Lett. 2024. PMID: 39321913 - Porcine Circovirus Type 2 Hijacks Host IPO5 to Sustain the Intracytoplasmic Stability of Its Capsid Protein.
Lin C, Hu J, Dai Y, Zhang H, Xu K, Dong W, Yan Y, Peng X, Zhou J, Gu J. Lin C, et al. J Virol. 2022 Dec 14;96(23):e0152222. doi: 10.1128/jvi.01522-22. Epub 2022 Nov 21. J Virol. 2022. PMID: 36409110 Free PMC article. - An ivermectin - atorvastatin combination impairs nuclear transport inhibiting dengue infection in vitro and in vivo.
Palacios-Rápalo SN, Farfan-Morales CN, Cordero-Rivera CD, De Jesús-González LA, Reyes-Ruiz JM, Meraz-Ríos MA, Del Ángel RM. Palacios-Rápalo SN, et al. iScience. 2023 Oct 27;26(12):108294. doi: 10.1016/j.isci.2023.108294. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034354 Free PMC article.
References
- Hung MC & Link W (2011) Protein localization in disease and therapy. J Cell Sci 124, 3381–3392. - PubMed
- Turner JG & Sullivan DM (2008) CRM1‐mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem 15, 2648–2655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous