The Effects of Stress Exposure on Prefrontal Cortex: Translating Basic Research into Successful Treatments for Post-Traumatic Stress Disorder - PubMed (original) (raw)
The Effects of Stress Exposure on Prefrontal Cortex: Translating Basic Research into Successful Treatments for Post-Traumatic Stress Disorder
Amy F T Arnsten et al. Neurobiol Stress. 2015.
Abstract
Research on the neurobiology of the stress response in animals has led to successful new treatments for Post-Traumatic Stress Disorder (PTSD) in humans. Basic research has found that high levels of catecholamine release during stress rapidly impair the top-down cognitive functions of the prefrontal cortex (PFC), while strengthening the emotional and habitual responses of the amygdala and basal ganglia. Chronic stress exposure leads to dendritic atrophy in PFC, dendritic extension in the amygdala, and strengthening of the noradrenergic (NE) system. High levels of NE release during stress engage low affinity alpha-1 adrenoceptors, (and likely beta-1 adrenoceptors), which rapidly reduce the firing of PFC neurons, but strengthen amygdala function. In contrast, moderate levels of NE release during nonstress conditions engage higher affinity alpha-2A receptors, which strengthen PFC, weaken amygdala, and regulate NE cell firing. Thus, either alpha-1 receptor blockade or alpha-2A receptor stimulation can protect PFC function during stress. Patients with PTSD have signs of PFC dysfunction. Clinical studies have found that blocking alpha-1 receptors with prazosin, or stimulating alpha-2A receptors with guanfacine or clonidine can be useful in reducing the symptoms of PTSD. Placebo-controlled trials have shown that prazosin is helpful in veterans, active duty soldiers and civilians with PTSD, including improvement of PFC symptoms such as impaired concentration and impulse control. Open label studies suggest that guanfacine may be especially helpful in treating children and adolescents who have experienced trauma. Thus, understanding the neurobiology of the stress response has begun to help patients with stress disorders.
Keywords: clonidine; dopamine; guanfacine; norepinephrine; pediatric; prazosin.
Figures
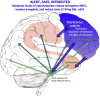
Fig. 1
During nonstressed arousal conditions when the subject is alert, safe and interested, the highly evolved prefrontal cortex (highlight in blue) provides top-down regulation of behavior, thought and emotion. It orchestrates behavioral response through extensive connections, e.g. to the amygdala, basal ganglia and brainstem, including the catecholamine neurons. Under these arousal conditions, there are moderate levels of catecholamine release, and phasic firing of LC neurons to appropriate stimuli (Rajkowski et al., 1998). Moderate levels of NE engage high affinity alpha-2A receptors, which strengthen PFC, but weaken amygdala (Arnsten, 2000). Alpha-2A receptors also reduce the tonic firing of LC neurons. All of these actions promote thoughtful PFC regulation of brain and behavior. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
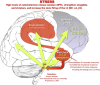
Fig. 2
Under conditions of uncontrollable stress, there are high levels of catecholamine release in brain, which weaken PFC function but strengthen the affective responses of the amygdala and the habitual responses of the basal ganglia. The amygdala activates the catecholamine under conditions of psychological stress, in addition to coordinating other aspects of the stress response (e.g. projections to the periaqueductal gray). Amygdala activation of the locus coeruleus via CRF increases tonic firing. High levels of NE release engage lower affinity alpha-1 and beta receptors, which enhance amygdala and weaken PFC function, thus producing a vicious cycle that maintains primitive circuits in control of behavior.
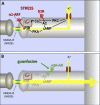
Fig. 3
The strength of dlPFC glutamate NMDA receptor connections on spines is dynamically modulated by arousal state. A. During stress, high levels of DA D1 receptor stimulation activate feedforward, cAMP- calcium (Ca2+) signaling, which opens nearby potassium (K+) channels to rapidly weaken connections and reduce PFC neuronal firing. NE stimulation of alpha-1 receptors also reduces PFC neuronal firing through IP3- Ca2+-protein kinase C (PKC) mechanisms (the ultrastructural localization of alpha-1 receptors in primate PFC is not yet known). Calcium is stored in the spine apparatus (indicated by asterisk), the endoplasmic reticulum in the spine. B. Guanfacine stimulation of post-synaptic alpha-2A receptors on PFC spines strengthens network connections, increases PFC neuronal firing and improves working memory by inhibiting feedforward cAMP- Ca2+ signaling and closing K+ channels on spines near the synapse. These actions likely contribute to guanfacine's therapeutic effects in patients. Additional abbreviations: AC = adenylyl cyclase; IP3R = inositol triphosphate receptors; PKA = protein kinase A.
Similar articles
- Chronic Stimulation of Alpha-2A-Adrenoceptors With Guanfacine Protects Rodent Prefrontal Cortex Dendritic Spines and Cognition From the Effects of Chronic Stress.
Hains AB, Yabe Y, Arnsten AF. Hains AB, et al. Neurobiol Stress. 2015;2:1-9. doi: 10.1016/j.ynstr.2015.01.001. Neurobiol Stress. 2015. PMID: 25664335 Free PMC article. - Guanfacine's mechanism of action in treating prefrontal cortical disorders: Successful translation across species.
Arnsten AFT. Arnsten AFT. Neurobiol Learn Mem. 2020 Dec;176:107327. doi: 10.1016/j.nlm.2020.107327. Epub 2020 Oct 17. Neurobiol Learn Mem. 2020. PMID: 33075480 Free PMC article. Review. - Loss of Prefrontal Cortical Higher Cognition with Uncontrollable Stress: Molecular Mechanisms, Changes with Age, and Relevance to Treatment.
Datta D, Arnsten AFT. Datta D, et al. Brain Sci. 2019 May 17;9(5):113. doi: 10.3390/brainsci9050113. Brain Sci. 2019. PMID: 31108855 Free PMC article. Review. - Stress weakens prefrontal networks: molecular insults to higher cognition.
Arnsten AF. Arnsten AF. Nat Neurosci. 2015 Oct;18(10):1376-85. doi: 10.1038/nn.4087. Epub 2015 Sep 25. Nat Neurosci. 2015. PMID: 26404712 Free PMC article. Review. - Adrenergic targets for the treatment of cognitive deficits in schizophrenia.
Arnsten AF. Arnsten AF. Psychopharmacology (Berl). 2004 Jun;174(1):25-31. doi: 10.1007/s00213-003-1724-3. Epub 2003 Dec 19. Psychopharmacology (Berl). 2004. PMID: 15205875 Review.
Cited by
- Chronic Stimulation of Alpha-2A-Adrenoceptors With Guanfacine Protects Rodent Prefrontal Cortex Dendritic Spines and Cognition From the Effects of Chronic Stress.
Hains AB, Yabe Y, Arnsten AF. Hains AB, et al. Neurobiol Stress. 2015;2:1-9. doi: 10.1016/j.ynstr.2015.01.001. Neurobiol Stress. 2015. PMID: 25664335 Free PMC article. - Psychosocial intervention in palliative care: What do psychologists need to know.
Feldstain A. Feldstain A. J Health Psychol. 2024 Jun;29(7):707-720. doi: 10.1177/13591053231222848. Epub 2024 Jan 28. J Health Psychol. 2024. PMID: 38282369 Free PMC article. Review. - The association between latent trauma and brain structure in children.
Jeong HJ, Durham EL, Moore TM, Dupont RM, McDowell M, Cardenas-Iniguez C, Micciche ET, Berman MG, Lahey BB, Kaczkurkin AN. Jeong HJ, et al. Transl Psychiatry. 2021 Apr 24;11(1):240. doi: 10.1038/s41398-021-01357-z. Transl Psychiatry. 2021. PMID: 33895776 Free PMC article. - Identification of biotypes in Attention-Deficit/Hyperactivity Disorder, a report from a randomized, controlled trial.
Leikauf JE, Griffiths KR, Saggar M, Hong DS, Clarke S, Efron D, Tsang TW, Hermens DF, Kohn MR, Williams LM. Leikauf JE, et al. Pers Med Psychiatry. 2017 Jul;3:8-17. doi: 10.1016/j.pmip.2017.02.001. Epub 2017 Mar 18. Pers Med Psychiatry. 2017. PMID: 35637915 Free PMC article. - Impact of Perceived Stress and Immune Status on Decision-Making Abilities during COVID-19 Pandemic Lockdown.
Tarantino V, Tasca I, Giannetto N, Mangano GR, Turriziani P, Oliveri M. Tarantino V, et al. Behav Sci (Basel). 2021 Dec 2;11(12):167. doi: 10.3390/bs11120167. Behav Sci (Basel). 2021. PMID: 34940102 Free PMC article.
References
- Amat J., Paul E., Zarza C., Watkins L.R., Maier S.F. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. - PMC - PubMed
- Arnsten A.F.T. The biology of feeling frazzled. Science. 1998;280:1711–1712. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous