Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis - PubMed (original) (raw)
Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis
Snehlata Kumari et al. Elife. 2014.
Abstract
Linear Ubiquitin chain Assembly Complex (LUBAC) is an E3 ligase complex that generates linear ubiquitin chains and is important for tumour necrosis factor (TNF) signaling activation. Mice lacking Sharpin, a critical subunit of LUBAC, spontaneously develop inflammatory lesions in the skin and other organs. Here we show that TNF receptor 1 (TNFR1)-associated death domain (TRADD)-dependent TNFR1 signaling in epidermal keratinocytes drives skin inflammation in Sharpin-deficient mice. Epidermis-restricted ablation of Fas-associated protein with death domain (FADD) combined with receptor-interacting protein kinase 3 (RIPK3) deficiency fully prevented skin inflammation, while single RIPK3 deficiency only delayed and partly ameliorated lesion development in Sharpin-deficient mice, showing that inflammation is primarily driven by TRADD- and FADD-dependent keratinocyte apoptosis while necroptosis plays a minor role. At the cellular level, Sharpin deficiency sensitized primary murine keratinocytes, human keratinocytes, and mouse embryonic fibroblasts to TNF-induced apoptosis. Depletion of FADD or TRADD in Sharpin-deficient HaCaT cells suppressed TNF-induced apoptosis, indicating the importance of FADD and TRADD in Sharpin-dependent anti-apoptosis signaling in keratinocytes.
Keywords: LUBAC; Sharpin; TNFR1; apoptosis; cell biology; immunology; mouse; skin inflammation; ubiquitin.
Conflict of interest statement
ID: Reviewing editor, eLife.
The other authors declare that no competing interests exist.
Figures
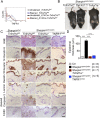
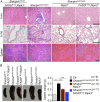
Figure 1.. Tumor necrosis factor receptor 1 (TNFR1) signaling in keratinocytes triggers chronic proliferative dermatitis phenotype in Sharpin cpdm/cpdm mice.
(A) Flow cytometric analysis of TNFR1 expression on the isolated keratinocytes from mice with the indicated genotypes. (B and C) Macroscopic pictures, Hematoxylin and Eosin staining (H&E), Keratin 6, 14, 10 and Loricrin as well as cleaved caspase-3 and F4/80 staining of the skin sections from 14-week-old littermate mice of the indicated genotypes. The scale bars are 100 μm. (D) Microscopic quantification of the epidermal thickness from 12–18-week-old mice of the indicated genotypes and their littermate controls (Ctr), which consisted of the following genotypes: Sharpin cpdm/wt;Tnfrsf1a −/−, Sharpin wt/wt;Tnfrsf1a −/−, Sharpin cpdm/wt;Tnfrsf1a fl/fl, Sharpin cpdm/wt;TNFR1E-KO, and Sharpin wt/wt;TNFR1E-KO. The Sharpin cpdm/cpdm group consisted of Sharpin cpdm/cpdm;Tnfrsf1a fl/fl and Sharpin cpdm/cpdm;Tnfrsf1a fl/wt mice that were littermates of the Sharpin cpdm/cpdm;TNFR1E-KO mice. The Sharpin cpdm/cpdm;Tnfrsf1a −/− mice were derived from a different line and shown here is the picture and the staining from the age-matched mice. Bars represent mean values ± SEM. Statistical significance was determined using the Student's t test (***p ≤ 0.001). DOI:
http://dx.doi.org/10.7554/eLife.03422.003
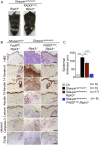
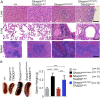
Figure 2.. Fas-associated protein with death domain (FADD) deficiency in keratinocytes prevents skin inflammation in Sharpin cpdm/cpdm mice.
(A and B) Macroscopic pictures, Hematoxylin and Eosin staining (H&E), Keratin 6, 14, 10 and Loricrin as well as cleaved caspase-3 and F4/80 staining of skin sections from 14-week-old mice of the indicated genotypes. The scale bars are 100 μm. (C) Microscopic quantification of the epidermal thickness from 12–18-week-old mice of the indicated genotypes and their littermate controls (Ctr), which consisted of the following genotypes: Sharpin wt/wt;Tnfrsf1a fl/fl, Sharpin cpdm/wt;Fadd fl/fl;Ripk3 −/−, Sharpin wt/wt;Fadd fl/fl;Ripk3 −/−, Sharpin cpdm/wt;FADDE-KO;Ripk3 −/− and Sharpin wt/wt;FADDE-KO;Ripk3 −/−. The Sharpin cpdm/cpdm group consisted of Sharpin cpdm/cpdm;Tnfrsf1a fl/fl and Sharpin cpdm/cpdm;Tnfrsf1a fl/wt mice that were littermates of the Sharpin cpdm/cpdm;TNFR1E-KO mice. Sharpin cpdm/cpdm;Ripk3 −/− mice were derived from the same breeding line as Sharpin cpdmcpdm;FADDE-KO;Ripk3 −/− mice and consisted of the genotype Sharpin cpdm/cpdm;Fadd fl/fl;Ripk3 −/−. Bars represent mean values ± SEM. Statistical significance was determined using the Student's t test (***p ≤ 0.001, **p ≤ 0.01). DOI:
http://dx.doi.org/10.7554/eLife.03422.004
Figure 2—figure supplement 1.. Variability among Sharpin cpdm/cpdm;Ripk3 −/− mice at a similar age.
The gross appearance of severe skin lesions in two Sharpin cpdm/cpdm;Ripk3 −/− mice at the age of 19 weeks (left) and a Sharpin cpdm/cpdm;Ripk3 −/− mouse at the same age with only a very mild phenotype (right). DOI:
http://dx.doi.org/10.7554/eLife.03422.005
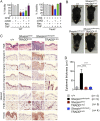
Figure 3.. Tumor necrosis factor receptor 1-associated death domain (TRADD) deficiency in keratinocytes prevents skin inflammation in Sharpin cpdm/cpdm mice.
(A) The percentage viability of wild type (WT) mouse embryonic fibroblasts (MEFs) (n = 3) and TRADD-deficient MEFs (n = 3) upon tumor necrosis factor (TNF), cycloheximide (CHX), caspase inhibitor (zVAD), and Necrostatin-1 (Nec) treatment alone or in combination for 20 hr and measurement by WST-1 assay. Bars represent average cell viability (± SD) of three independent experiments. (B and C) Macroscopic gross appearance of the WT (+/+) and littermate mice of the indicated genotype at the age of 12 weeks (B) and (H&E), Keratin 6, 14, 10 and Loricrin as well as cleaved caspase-3 and F4/80 staining of the skin sections from 12-week-old mice of the indicated genotypes (C). Scale bars in (C) are 100 μm. (D) Microscopic quantification of the epidermal thickness from 12-week-old littermate mice of Sharpin cpdm/cpdm, Sharpin wt/wt;TRADDE-KO, Sharpin cpdm/cpdm;TRADDE-KO and age-matched WT (+/+) is shown. Bars represent mean values ± SD. Statistical significance was determined using the one-way ANOVA test (****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.006
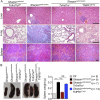
Figure 4.. Tumor necrosis factor receptor 1 (TNFR1) deficiency in Sharpin cpdm/cpdm mice rescues the inflammation of lung, liver and splenomegaly but not epidermal keratinocyte-restricted knockout of TNFR1.
(A and B) H&E staining of liver, lung and spleen, and macroscopic pictures of spleen from mice with the indicated genotypes as well as measurement of spleen weight from 12–18-week-old Sharpin cpdm/cpdm, Sharpin cpdm/cpdm;Tnfrsf1a −/−, and Sharpin cpdm/cpdm;TNFR1E-KO mice and their littermate controls (Ctr), which consisted of the following genotypes: Sharpin cpdm/wt;Tnfrsf1a −/−, Sharpin wt/wt;Tnfrsf1a −/−, Sharpin cpdm/wt;Tnfrsf1a fl/fl, Sharpin cpdm/wt;TNFR1E-KO, and Sharpin wt/wt;TNFR1E-KO. The Sharpin cpdm/cpdm group consisted of Sharpin cpdm/cpdm;Tnfrsf1a fl/fl and Sharpin cpdm/cpdm;Tnfrsf1a fl/wt mice that were littermates of the Sharpin cpdm/cpdm;TNFR1E-KO mice. Scale bars in (A) are 100 μm. Results are expressed as mean values ± SEM. Statistical significance was determined using unpaired Student's t test (two-tailed) (***p ≤ 0.001). DOI:
http://dx.doi.org/10.7554/eLife.03422.007
Figure 5.. Receptor-interacting protein kinase 3 (_Ripk3_−/−) and epidermis-specific Fas-associated protein with death domain (FADD) together with _Ripk3_−/− (FADDE-KO;Ripk3 −/−) in Sharpin cpdm/cpdm mice partially rescues the inflammation of the lung, liver, and splenomegaly.
(A and B) H&E staining of liver, lung and spleen, and macroscopic pictures of spleen from mice with the indicated genotypes as well as measurement of spleen weight from 12–18-week-old Sharpin cpdm/cpdm, Sharpin cpdm/cpdm;Ripk3 −/− and Sharpin cpdm/cpdm;FADDE-KO;Ripk3 −/− mice and their littermate controls (Ctr), which consisted of the following genotypes: Sharpin wt/wt;Tnfrsf1a fl/fl, Sharpin cpdm/wt;Fadd fl/fl;Ripk3 −/−, Sharpin wt/wt;Fadd fl/fl;Ripk3 −/−, Sharpin cpdm/wt;FADDE-KO;Ripk3 −/−, and Sharpin wt/wt;FADDE-KO;Ripk3 −/−. The Sharpin cpdm/cpdm group consisted of Sharpin cpdm/cpdm;Tnfrsf1a fl/fl and Sharpin cpdm/cpdm;Tnfrsf1a fl/wt mice that were littermates of the Sharpin cpdm/cpdm;TNFR1E-KO mice. The Sharpin cpdm/cpdm;Ripk3 −/− mice were derived from the same breeding line as Sharpin cpdmcpdm;FADDE-KO;Ripk3 −/− mice and consisted of the genotype Sharpin cpdm/cpdm;Fadd fl/fl;Ripk3 −/−. Scale bars in (A) are 100 μm. Results are expressed as mean values ± SEM. Statistical significance was determined using unpaired Student's t test (two-tailed) (***p ≤ 0.001). DOI:
http://dx.doi.org/10.7554/eLife.03422.008
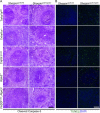
Figure 5—figure supplement 1.. Cell death in the spleen of Sharpin cpdm/cpdm, Sharpin cpdm/cpdm; Tnfrsf1a −/−, Sharpin cpdm/cpdm;TNFR1E-KO, Sharpin cpdm/cpdm;Ripk3 −/− and Sharpin cpdm/cpdm;FADDE-KO;Ripk3 −/− mice.
(A and B) Cleaved caspase-3 (A) and TUNEL (B) staining on the spleen tissue sections from mice with the indicated genotypes between 12–18 weeks. The control mice compared were from the same breeding but not always littermates. Representative images are shown. The scale bars are 100 μm. Nuclei in (B) were visualized using DAPI. DOI:
http://dx.doi.org/10.7554/eLife.03422.009
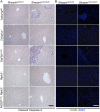
Figure 5—figure supplement 2.. Cell death in the liver of Sharpin cpdm/cpdm, Sharpin cpdm/cpdm; Tnfrsf1a −/−, Sharpin cpdm/cpdm;TNFR1E-KO, Sharpin cpdm/cpdm;Ripk3 −/− and Sharpin cpdm/cpdm;FADDE-KO;Ripk3 −/− mice.
(A and B) Cleaved caspase-3 (A) and TUNEL (B) staining of liver tissue sections from mice with the indicated genotypes between 12–18 weeks. The control mice compared were from the same breeding but not always littermates. Representative images are shown. The scale bars are 100 μm. Nuclei in (B) were visualized using DAPI. DOI:
http://dx.doi.org/10.7554/eLife.03422.010
Figure 5—figure supplement 3.. Cell death in the lung of Sharpin cpdm/cpdm, Sharpin cpdm/cpdm; Tnfrsf1a −/−, Sharpin cpdm/cpdm;TNFR1E-KO, Sharpin cpdm/cpdm;Ripk3 −/− and Sharpin cpdm/cpdm;FADDE-KO;Ripk3 −/− mice.
(A and B) Cleaved caspase-3 (A) and TUNEL (B) staining of lung tissue sections from mice with the indicated genotypes between 12–18 weeks. The control mice compared were from the same breeding but not always littermates. Representative images are shown. The scale bars are 100 μm. Nuclei in (B) were visualized using DAPI. DOI:
http://dx.doi.org/10.7554/eLife.03422.011
Figure 6.. Epidermal keratinocyte-restricted knockout of tumor necrosis factor receptor 1-associated death domain (TRADD) (TRADDE-KO) in Sharpin cpdm/cpdm mice has a minor effect on the inflammation of the lung, liver, and splenomegaly.
(A and B) H&E staining of liver, lung, and spleen and macroscopic pictures of spleen as well as measurement of spleen weight from mice with the indicated genotypes derived from wild type (+/+), Sharpin cpdm/cpdm, TRADDE-KO or Sharpin cpdm/cpdm;TRADDE-KO mice at the age of 12 weeks. The mice used here are the littermates. Scale bars in (A) are 100 μm. Results are expressed as mean values ± SD. Statistical significance was determined using ANOVA test (***p ≤ 0.001 and ****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.012
Figure 7.. Sharpin regulates tumor necrosis factor (TNF)-induced apoptosis signaling cascade in mouse embryonic fibroblasts (MEFs).
(A–D) TNF- and cycloheximide (CHX)-induced apoptosis in wild type (+/+) or Sharpin cpdm/cpdm MEFs. Apoptosis in MEFs stimulated with TNF (10 ng/ml) and CHX (1 μg/ml) for 4 hr was examined by immunofluorescent staining using α-cleaved caspase-3 antibody with Alexa488 conjugated secondary antibody (A), by fluorescence-activated cell sorting (FACS) analysis using annexin V staining (B), by immunoblotting using α-cleaved caspase-3 and α-Poly (ADP-ribose) polymerase (PARP) antibodies (C), or by caspase-8 activity assay measured using a luminol-based assay. Scale bars in (A) are 100 μm. (E and F) TNF-induced apoptosis in MEFs analyzed by immunoblotting (E) or by caspase-8 activity assay (F) as in (C and D). Results are expressed as mean values ± SD. Statistical significance was determined using ANOVA test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.013
Figure 8.. Sharpin regulates tumor necrosis factor (TNF)-induced apoptosis signaling cascade in HaCaT cells.
(A) Immunoblotting of stable knockdown Sharpin in HaCaT cells using α-Sharpin antibody. Control shRNA (Ctr) was used for the control knockdown. α-Vinculin antibody was used for the loading control. (B) Fluorescence-activated cell sorting (FACS) analysis of annexin V staining in parental, Ctr, and Sharpin knockdown HaCaT cells, stimulated with TNF (100 ng/ml) for 16 hr or TNF with cycloheximide (CHX) (1 μg/ml) for 6 hr. (C) Caspase-8 activity measurement using a luminol-based assay upon stimulation with TNF alone, or TNF and CHX for the indicated time in Ctr and Sharpin knockdown HaCaT cells. (D) Caspase-8 activity measurement upon stimulation with TNF, TNF + CHX with or without Necrostatin-1 (Nec) (30 μmol) for 6 hr in Ctr and Sharpin knockdown HaCaT cells. Results are expressed as mean values ± SD. Statistical significance was determined using ANOVA test (****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.014
Figure 8—figure supplement 1.. A LUBAC component, HOIL-1L interacting protein (HOIP), plays a role in tumor necrosis factor (TNF)-induced apoptosis in HaCaT cells.
(A–D) Immunoblot of HOIP (A) after stable knocked down of HOIP in HaCaT cells by shRNA. α-Tubulin antibody was used for the loading control. Fluorescence-activated cell sorting (FACS) analysis of annexin V positive cells after stimulation of control (Ctr) and HOIP knockdown HaCaT cells with TNF alone or TNF + cycloheximide (CHX) (B) and caspase-8 activity assay (B and D) after stimulation of Ctr and HOIP knockdown HaCaT cells with TNF alone (C) or TNF + CHX (D). Results are expressed as mean values ± SD. Statistical significance was determined using ANOVA test (**p ≤ 0.01, ****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.015
Figure 9.. Fas-associated protein with death domain (FADD)- and tumor necrosis factor receptor 1-associated death domain (TRADD)-dependent enhanced sensitivity of Sharpin-deficient keratinocytes to tumor necrosis factor (TNF)-induced apoptosis.
(A) Percentage viability of primary keratinocytes isolated from Sharpin cpdm/cpdm, Sharpin cpdm/cpdm;Ripk3 −/−, Sharpin cpdm/cpdm;FADDE-KO;Ripk3 −/− and Sharpin cpdm/cpdm;TNFR1E-KO, and their respective control pups (n = 2) upon treatment with increasing TNF concentration (20, 50 and 100 ng/ml) in the presence or absence of cycloheximide (CHX) (1 μg/ml) for 24 hr. Viability of TNF-treated cells was normalized over their untreated control cells, and viability of TNF + CHX-treated cells was normalized over their CHX-treated control cells. The result shown here is representative of two independent experiments. The percentage viability was assessed using the WST-1 assay. Bars represent mean values ± SEM. Statistical significance was determined using the Student's t test (**p ≤ 0.01, *p ≤ 0.05). (B–G) Sharpin with FADD (B) or TRADD (E) was stably knocked down in HaCaT cells. Knockdown efficiency was analyzed by immunoblotting using α-Sharpin, α-FADD, and α-TRADD antibodies. Caspase-8 activity measurement in Sharpin knockdown, FADD knockdown, and double knockdown of Sharpin and FADD HaCaT cells (C and D) as well as Sharpin knockdown, TRADD knockdown, and double knockdown of Sharpin and TRADD HaCaT cells (F and G) upon treatment with TNF alone (C and F) or TNF + CHX (D and G) for the indicated time. Results are expressed as mean values ± SD. Statistical significance was determined using ANOVA test (****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.016
Figure 9—figure supplement 1.. Fas-associated protein with death domain (FADD) plays an important role in the Sharpin-dependent apoptosis signaling.
(A) Immunoblot of FADD after stable knocked down of FADD in wild type (+/+) mouse embryonic fibroblasts (MEFs) and Sharpin cpdm/cpdm MEFs by shRNA. α-Vinculin antibody was used for the loading control. (B) Fluorescence-activated cell sorting (FACS) analysis of annexin V positive cells after stimulation with tumor necrosis factor (TNF) + cycloheximide (CHX) for 4 hr in +/+ shCtr MEFs, +/+ shFADD MEFs, Sharpin cpdm/cpdm shCtr MEFs, and Sharpin cpdm/cpdm shFADD MEFs. (C) Caspase-8 activity measurement upon stimulation with TNF with or without CHX for the indicated time in +/+ shCtr MEFs, +/+ shFADD MEFs, Sharpin cpdm/cpdm shCtr MEFs and Sharpin cpdm/cpdm shFADD MEFs. Results are expressed as mean values ± SD. Statistical significance was determined using ANOVA test (****p ≤ 0.0001). DOI:
http://dx.doi.org/10.7554/eLife.03422.017
Similar articles
- TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice.
Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N, Lalaoui N, Lawlor KE, Vanyai H, Hall C, Bankovacki A, Gangoda L, Wong WW, Corbin J, Huang C, Mocarski ES, Murphy JM, Alexander WS, Voss AK, Vaux DL, Kaiser WJ, Walczak H, Silke J. Rickard JA, et al. Elife. 2014 Dec 2;3:e03464. doi: 10.7554/eLife.03464. Elife. 2014. PMID: 25443632 Free PMC article. - RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis.
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. Dannappel M, et al. Nature. 2014 Sep 4;513(7516):90-4. doi: 10.1038/nature13608. Epub 2014 Aug 17. Nature. 2014. PMID: 25132550 Free PMC article. - Redundant and receptor-specific activities of TRADD, RIPK1 and FADD in death receptor signaling.
Füllsack S, Rosenthal A, Wajant H, Siegmund D. Füllsack S, et al. Cell Death Dis. 2019 Feb 11;10(2):122. doi: 10.1038/s41419-019-1396-5. Cell Death Dis. 2019. PMID: 30741924 Free PMC article. - Poly-ubiquitination in TNFR1-mediated necroptosis.
Dondelinger Y, Darding M, Bertrand MJ, Walczak H. Dondelinger Y, et al. Cell Mol Life Sci. 2016 Jun;73(11-12):2165-76. doi: 10.1007/s00018-016-2191-4. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066894 Free PMC article. Review. - Pursuing different 'TRADDes': TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1.
Kieser A. Kieser A. Biol Chem. 2008 Oct;389(10):1261-71. doi: 10.1515/BC.2008.144. Biol Chem. 2008. PMID: 18713013 Review.
Cited by
- Keratinocyte-specific deletion of SHARPIN induces atopic dermatitis-like inflammation in mice.
Sundberg JP, Pratt CH, Goodwin LP, Silva KA, Kennedy VE, Potter CS, Dunham A, Sundberg BA, HogenEsch H. Sundberg JP, et al. PLoS One. 2020 Jul 20;15(7):e0235295. doi: 10.1371/journal.pone.0235295. eCollection 2020. PLoS One. 2020. PMID: 32687504 Free PMC article. - NF-κB inhibition in keratinocytes causes RIPK1-mediated necroptosis and skin inflammation.
Kumari S, Van TM, Preukschat D, Schuenke H, Basic M, Bleich A, Klein U, Pasparakis M. Kumari S, et al. Life Sci Alliance. 2021 Apr 15;4(6):e202000956. doi: 10.26508/lsa.202000956. Print 2021 Jun. Life Sci Alliance. 2021. PMID: 33858959 Free PMC article. - Autoinflammatory Skin Disorders: The Inflammasomme in Focus.
Gurung P, Kanneganti TD. Gurung P, et al. Trends Mol Med. 2016 Jul;22(7):545-564. doi: 10.1016/j.molmed.2016.05.003. Epub 2016 Jun 3. Trends Mol Med. 2016. PMID: 27267764 Free PMC article. Review. - The ubiquitin ligase HOIL-1L regulates immune responses by interacting with linear ubiquitin chains.
Gomez-Diaz C, Jonsson G, Schodl K, Deszcz L, Bestehorn A, Eislmayr K, Almagro J, Kavirayani A, Seida M, Fennell LM, Hagelkruys A, Kovarik P, Penninger JM, Ikeda F. Gomez-Diaz C, et al. iScience. 2021 Oct 8;24(11):103241. doi: 10.1016/j.isci.2021.103241. eCollection 2021 Nov 19. iScience. 2021. PMID: 34755089 Free PMC article. - A phase I, randomized, ascending-dose study to assess safety, pharmacokinetics, and activity of GDC-8264, a RIP1 inhibitor, in healthy volunteers.
Jones NS, Kshirsagar S, Mohanan V, Ramakrishnan V, Di Nucci F, Ma L, Mao J, Ding H, Klabunde S, Vucic D, Pan L, Lekkerkerker AN, Chen Y, Rothenberg ME. Jones NS, et al. Clin Transl Sci. 2023 Oct;16(10):1997-2009. doi: 10.1111/cts.13607. Epub 2023 Aug 24. Clin Transl Sci. 2023. PMID: 37596814 Free PMC article. Clinical Trial.
References
- Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ. RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. Journal of Immunology. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. - DOI - PMC - PubMed
- Chen NJ, Chio, Lin WJ, Duncan G, Chau H, Katz D, Huang HL, Pike KA, Hao Z, Su YW, Yamamoto K, de Pooter RF, Zúñiga-Pflücker JC, Wakeham A, Yeh WC, Mak TW. Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors. Proceedings of the National Academy of Sciences of USA. 2008;105:12429–34. doi: 10.1073/pnas.0806585105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous