Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial - PubMed (original) (raw)
Clinical Trial
. 2015 May 1;33(13):1430-7.
doi: 10.1200/JCO.2014.59.0703. Epub 2014 Dec 1.
Brian I Rini 2, David F McDermott 2, Bruce G Redman 2, Timothy M Kuzel 2, Michael R Harrison 2, Ulka N Vaishampayan 2, Harry A Drabkin 2, Saby George 2, Theodore F Logan 2, Kim A Margolin 2, Elizabeth R Plimack 2, Alexandre M Lambert 2, Ian M Waxman 2, Hans J Hammers 2
Affiliations
- PMID: 25452452
- PMCID: PMC4806782
- DOI: 10.1200/JCO.2014.59.0703
Clinical Trial
Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial
Robert J Motzer et al. J Clin Oncol. 2015.
Abstract
Purpose: Nivolumab is a fully human immunoglobulin G4 programmed death-1 immune checkpoint inhibitor antibody that restores T-cell immune activity. This phase II trial assessed the antitumor activity, dose-response relationship, and safety of nivolumab in patients with metastatic renal cell carcinoma (mRCC).
Patients and methods: Patients with clear-cell mRCC previously treated with agents targeting the vascular endothelial growth factor pathway were randomly assigned (blinded ratio of 1:1:1) to nivolumab 0.3, 2, or 10 mg/kg intravenously once every 3 weeks. The primary objective was to evaluate the dose-response relationship as measured by progression-free survival (PFS); secondary end points included objective response rate (ORR), overall survival (OS), and safety.
Results: A total of 168 patients were randomly assigned to the nivolumab 0.3- (n = 60), 2- (n = 54), and 10-mg/kg (n = 54) cohorts. One hundred eighteen patients (70%) had received more than one prior systemic regimen. Median PFS was 2.7, 4.0, and 4.2 months, respectively (P = .9). Respective ORRs were 20%, 22%, and 20%. Median OS was 18.2 months (80% CI, 16.2 to 24.0 months), 25.5 months (80% CI, 19.8 to 28.8 months), and 24.7 months (80% CI, 15.3 to 26.0 months), respectively. The most common treatment-related adverse event (AE) was fatigue (24%, 22%, and 35%, respectively). Nineteen patients (11%) experienced grade 3 to 4 treatment-related AEs.
Conclusion: Nivolumab demonstrated antitumor activity with a manageable safety profile across the three doses studied in mRCC. No dose-response relationship was detected as measured by PFS. These efficacy and safety results in mRCC support study in the phase III setting.
Trial registration: ClinicalTrials.gov NCT01354431.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
Fig 1.
Patient disposition (as of May 15, 2013, data cutoff). (*) One patient not treated; no longer met study criteria. (†) Includes patients continuing in treatment period and patients in follow-up period.
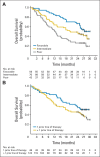
Fig 2.
(A) Progression-free and (B) overall survival by treatment arm (randomly assigned patients). Tick marks represent censored observations.
Fig 3.
Duration of response in patients who achieved objective response by dose treatment arm. Based on data cutoff date of March 5, 2014.
Fig 4.
Overall survival (randomly assigned patients) by (A) Memorial Sloan-Kettering Cancer Center risk group and (B) number of prior therapies in advanced or metastatic setting. Tick marks represent censored observations.
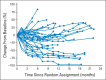
Fig A1.
Changes in measurable lesions from study baseline in patients treated beyond progression (n = 36 [0.3 mg/kg, n = 10; 2 mg/kg, n = 12; 10 mg/kg, n = 14]). Circles represent assessments that occurred after initial progression.
Similar articles
- Safety and Efficacy of Nivolumab in Patients With Metastatic Renal Cell Carcinoma Treated Beyond Progression: A Subgroup Analysis of a Randomized Clinical Trial.
George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM, Rini BI. George S, et al. JAMA Oncol. 2016 Sep 1;2(9):1179-86. doi: 10.1001/jamaoncol.2016.0775. JAMA Oncol. 2016. PMID: 27243803 Free PMC article. Clinical Trial. - Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma.
Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, Fountzilas G, Peschel C, Flodgren P, Maneval EC, Chen I, Vogelzang NJ. Escudier B, et al. J Clin Oncol. 2009 Sep 1;27(25):4068-75. doi: 10.1200/JCO.2008.20.5476. Epub 2009 Aug 3. J Clin Oncol. 2009. PMID: 19652072 Clinical Trial. - Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study.
Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA, Kollmannsberger C, Heng DYC, Spratlin J, McHenry MB, Amin A. Hammers HJ, et al. J Clin Oncol. 2017 Dec 1;35(34):3851-3858. doi: 10.1200/JCO.2016.72.1985. Epub 2017 Jul 5. J Clin Oncol. 2017. PMID: 28678668 Free PMC article. Clinical Trial. - Spotlight on nivolumab in the treatment of renal cell carcinoma: design, development, and place in therapy.
Venur VA, Joshi M, Nepple KG, Zakharia Y. Venur VA, et al. Drug Des Devel Ther. 2017 Apr 11;11:1175-1182. doi: 10.2147/DDDT.S110209. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28442891 Free PMC article. Review. - Safety and efficacy of nivolumab therapy in patients with metastatic renal cell carcinoma and impaired kidney function.
Sengul N, Gültürk I, Yilmaz M, Celik E, Paksoy N, Yekedüz E, Ürün Y, Basaran M, Özgüroğlu M. Sengul N, et al. Actas Urol Esp (Engl Ed). 2024 May;48(4):273-280. doi: 10.1016/j.acuroe.2024.04.002. Epub 2024 Apr 1. Actas Urol Esp (Engl Ed). 2024. PMID: 38570033 Review. English, Spanish.
Cited by
- Checkpoint modulation--A new way to direct the immune system against renal cell carcinoma.
Bedke J, Kruck S, Gakis G, Stenzl A, Goebell PJ. Bedke J, et al. Hum Vaccin Immunother. 2015;11(5):1201-8. doi: 10.1080/21645515.2015.1016657. Hum Vaccin Immunother. 2015. PMID: 25912622 Free PMC article. Review. - An immunotherapy response prediction model derived from proliferative CD4+ T cells and antigen-presenting monocytes in ccRCC.
Zheng K, Gao L, Hao J, Zou X, Hu X. Zheng K, et al. Front Immunol. 2022 Aug 25;13:972227. doi: 10.3389/fimmu.2022.972227. eCollection 2022. Front Immunol. 2022. PMID: 36091022 Free PMC article. - Model Informed Dosing Regimen and Phase I Results of the Anti-PD-1 Antibody Budigalimab (ABBV-181).
Powderly J, Spira A, Kondo S, Doi T, Luke JJ, Rasco D, Gao B, Tanner M, Cassier PA, Gazzah A, Italiano A, Tosi D, Afar DE, Parikh A, Engelhardt B, Englert S, Lambert SL, Kasichayanula S, Mensing S, Menon R, Vosganian G, Tolcher A. Powderly J, et al. Clin Transl Sci. 2021 Jan;14(1):277-287. doi: 10.1111/cts.12855. Epub 2020 Dec 26. Clin Transl Sci. 2021. PMID: 32770720 Free PMC article. Clinical Trial. - Quantifying Treatment Benefit in Molecular Subgroups to Assess a Predictive Biomarker.
Iasonos A, Chapman PB, Satagopan JM. Iasonos A, et al. Clin Cancer Res. 2016 May 1;22(9):2114-20. doi: 10.1158/1078-0432.CCR-15-2517. Clin Cancer Res. 2016. PMID: 27141007 Free PMC article. Review. - Exploration of the role of Cuproptosis genes and their related long non-coding RNA in clear cell renal cell carcinoma: a comprehensive bioinformatics study.
Xia D, Liu Q, Jiao W, Peng L, Wang Q, Tuo Z, Bi L. Xia D, et al. BMC Cancer. 2022 Nov 6;22(1):1141. doi: 10.1186/s12885-022-10278-z. BMC Cancer. 2022. PMID: 36335291 Free PMC article.
References
- Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii65–vii71. - PubMed
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. - PubMed
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials