Mast cell mediators: their differential release and the secretory pathways involved - PubMed (original) (raw)
Review
Mast cell mediators: their differential release and the secretory pathways involved
Tae Chul Moon et al. Front Immunol. 2014.
Abstract
Mast cells (MC) are widely distributed throughout the body and are common at mucosal surfaces, a major host-environment interface. MC are functionally and phenotypically heterogeneous depending on the microenvironment in which they mature. Although MC have been classically viewed as effector cells of IgE-mediated allergic diseases, they are also recognized as important in host defense, innate and acquired immunity, homeostatic responses, and immunoregulation. MC activation can induce release of pre-formed mediators such as histamine from their granules, as well as release of de novo synthesized lipid mediators, cytokines, and chemokines that play diverse roles, not only in allergic reactions but also in numerous physiological and pathophysiological responses. Indeed, MC release their mediators in a discriminating and chronological manner, depending upon the stimuli involved and their signaling cascades (e.g., IgE-mediated or Toll-like receptor-mediated). However, the precise mechanisms underlying differential mediator release in response to these stimuli are poorly known. This review summarizes our knowledge of MC mediators and will focus on what is known about the discriminatory release of these mediators dependent upon diverse stimuli, MC phenotypes, and species of origin, as well as on the intracellular synthesis, storage, and secretory processes involved.
Keywords: exocytosis; exosome; granule; lipid body; lysosome; secretion.
Figures
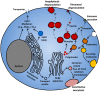
Figure 1
Mediator release from MC. MC release various mediators from different compartments following different stimuli. MC rapidly release pre-stored granule contents by piecemeal or anaphylactic degranulation. Immature progranules and mature granules can fuse with endosomes, and store lysosomal proteins. Some mediators can be released from granules and endosomes through exosomal secretion. Lipid mediators such as PGD2 and LTC4 are synthesized in lipid bodies, nuclear and ER membranes, and released through active transporters. De novo synthesized cytokines and chemokines packaged in secretory vesicles are released through constitutive exocytosis.
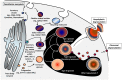
Figure 2
Model of genesis of MC secretory lysosomes (granules) and their heterogeneity/plasticity [adapted from Raposo et al. (43)]. Type I granules and type III granules are formed from lysosomal/endosomal pathway and by unit granule fusion from the trans-Golgi region, respectively. Secretory lysosomes that bud from trans-Golgi network contain MHC class II molecules, mannose-6-phosphate receptor (M6PR), and the lysosomal markers LAMP-1, -2, and β-hexosaminidase. It is postulated that post-endosomal, type II secretory lysosomes arise through the fusion of Type I and III granules. The relationship of this model to observations of heterogeneity of secretory lysosomes with regard to histamine or 5-HT content and VAMP-8 expression is unclear and there likely exists more granule heterogeneity/plasticity than three types (44). The mechanism of genesis of granule types is poorly understood (black area).
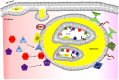
Figure 3
Model of lipid body biogenesis and structure. Neutral lipids synthesized in the ER accumulate between bilayer of ER membrane and bud off as a lipid body. Lipid bodies have phospholipid monolayer on their outside. However, lipid bodies contain a bilayer core structure inside, which provides a hydrophilic area. The bilayer core can be created by incorporation of multiple loops of ER membrane and explains how ER membrane proteins (e.g., caveolin-1 and ribosome) are incorporated into lipid bodies. However, the exact mechanism of formation of the bilayer structure is poorly understood. Enzymes required for eicosanoid production have been found in both outer membrane and core of lipid bodies. Increased intracellular Ca2+ after MC stimulation induces activation and translocation of cPLA2, 5-LO, and 15-LO to the lipid body membrane for eicosanoid synthesis. Further studies are required to unveil how MC control synthesis and secretion of arachidonic acid metabolites in a stimulus-specific fashion.
Similar articles
- Adaptor protein-3: A key player in RBL-2H3 mast cell mediator release.
da Silva EZ, Freitas-Filho EG, de Souza-Júnior DA, daSilva LL, Jamur MC, Oliver C. da Silva EZ, et al. PLoS One. 2017 Mar 8;12(3):e0173462. doi: 10.1371/journal.pone.0173462. eCollection 2017. PLoS One. 2017. PMID: 28273137 Free PMC article. - Involvement of Mast Cells in the Pathophysiology of Pain.
Mai L, Liu Q, Huang F, He H, Fan W. Mai L, et al. Front Cell Neurosci. 2021 Jun 10;15:665066. doi: 10.3389/fncel.2021.665066. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34177465 Free PMC article. - Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights.
Elieh Ali Komi D, Bjermer L. Elieh Ali Komi D, et al. Clin Rev Allergy Immunol. 2019 Apr;56(2):234-247. doi: 10.1007/s12016-018-8720-1. Clin Rev Allergy Immunol. 2019. PMID: 30506113 Review. - The tetraspanin CD63 is required for efficient IgE-mediated mast cell degranulation and anaphylaxis.
Kraft S, Jouvin MH, Kulkarni N, Kissing S, Morgan ES, Dvorak AM, Schröder B, Saftig P, Kinet JP. Kraft S, et al. J Immunol. 2013 Sep 15;191(6):2871-8. doi: 10.4049/jimmunol.1202323. Epub 2013 Aug 14. J Immunol. 2013. PMID: 23945142 Free PMC article. - The Role of Mast Cells in IgE-Independent Lung Diseases.
Komi DEA, Mortaz E, Amani S, Tiotiu A, Folkerts G, Adcock IM. Komi DEA, et al. Clin Rev Allergy Immunol. 2020 Jun;58(3):377-387. doi: 10.1007/s12016-020-08779-5. Clin Rev Allergy Immunol. 2020. PMID: 32086776 Free PMC article. Review.
Cited by
- p16Ink4a-induced senescence in cultured mast cells as a model for ageing reveals significant morphological and functional changes.
Kleeblatt E, Lazki-Hagenbach P, Nabet E, Cohen R, Bahri R, Rogers N, Langton A, Bulfone-Paus S, Frenkel D, Sagi-Eisenberg R. Kleeblatt E, et al. Immun Ageing. 2024 Nov 11;21(1):77. doi: 10.1186/s12979-024-00478-5. Immun Ageing. 2024. PMID: 39529115 Free PMC article. - Mast cells promote choroidal neovascularization in a model of age-related macular degeneration.
Dabouz R, Abram P, Rivera JC, Chemtob S. Dabouz R, et al. J Neuroinflammation. 2024 Oct 1;21(1):247. doi: 10.1186/s12974-024-03229-x. J Neuroinflammation. 2024. PMID: 39354493 Free PMC article. - Histaminergic System Activity in the Central Nervous System: The Role in Neurodevelopmental and Neurodegenerative Disorders.
Szukiewicz D. Szukiewicz D. Int J Mol Sci. 2024 Sep 12;25(18):9859. doi: 10.3390/ijms25189859. Int J Mol Sci. 2024. PMID: 39337347 Free PMC article. Review. - Emerging trends and research hotspots in the relationship between mast cells and atopic dermatitis based on the literature from 2001 to 2024: A bibliometric and visualized analysis.
Zuo W, Yue Z, Xu S, Sun CH, Zou XF, Ma J, Yan H, Gu XW, Wang MY. Zuo W, et al. Skin Res Technol. 2024 Sep;30(9):e70053. doi: 10.1111/srt.70053. Skin Res Technol. 2024. PMID: 39234634 Free PMC article. - Fascia as a regulatory system in health and disease.
Slater AM, Barclay SJ, Granfar RMS, Pratt RL. Slater AM, et al. Front Neurol. 2024 Aug 7;15:1458385. doi: 10.3389/fneur.2024.1458385. eCollection 2024. Front Neurol. 2024. PMID: 39188704 Free PMC article. Review.
References
- Schick B, Austen KF. Rat serosal mast cell degranulation mediated by chymase, an endogenous secretory granule protease: active site-dependent initiation at 1 degree C. J Immunol (1986) 136(10):3812–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources