A refined reaction-diffusion model of tau-microtubule dynamics and its application in FDAP analysis - PubMed (original) (raw)
A refined reaction-diffusion model of tau-microtubule dynamics and its application in FDAP analysis
Maxim Igaev et al. Biophys J. 2014.
Abstract
Fluorescence decay after photoactivation (FDAP) and fluorescence recovery after photobleaching (FRAP) are well established approaches for studying the interaction of the microtubule (MT)-associated protein tau with MTs in neuronal cells. Previous interpretations of FDAP/FRAP data have revealed dwell times of tau on MTs in the range of several seconds. However, this is difficult to reconcile with a dwell time recently measured by single-molecule analysis in neuronal processes that was shorter by two orders of magnitude. Questioning the validity of previously used phenomenological interpretations of FDAP/FRAP data, we have generalized the standard two-state reaction-diffusion equations by 1), accounting for the parallel and discrete arrangement of MTs in cell processes (i.e., homogeneous versus heterogeneous distribution of tau-binding sites); and 2), explicitly considering both active (diffusion upon MTs) and passive (piggybacking upon MTs at rates of slow axonal transport) motion of bound tau. For some idealized cases, analytical solutions were derived. By comparing them with the full numerical solution and Monte Carlo simulations, the respective validity domains were mapped. Interpretation of our FDAP data (from processes of neuronally differentiated PC12 cells) in light of the heterogeneous formalism yielded independent estimates for the association (∼2 ms) and dwell (∼100 ms) times of tau to/on a single MT rather than in an MT array. The dwell time was shorter by orders of magnitude than that in a previous report where a homogeneous topology of MTs was assumed. We found that the diffusion of bound tau was negligible in vivo, in contrast to an earlier report that tau diffuses along the MT lattice in vitro. Methodologically, our results demonstrate that the heterogeneity of binding sites cannot be ignored when dealing with reaction-diffusion of cytoskeleton-associated proteins. Physiologically, the results reveal the behavior of tau in cellular processes, which is noticeably different from that in vitro.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
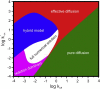
Figure 1
Superposition of the validity areas of the four simplified solutions. The regime space shows where each simplified solution approximates well the full numerical solution (the sum of squared residuals, res2, is <0.01, D = 10 _μ_m2/s, σ = 2 _μ_m). The area where none of the simplified solutions is suitable is assigned to the full numerical solution. While constructing the superposition, overlaps between different validity areas were assigned to the one for the simplified solution with the fewest number of fit parameters. To see this figure in color, go online.
Figure 2
Homogeneous and heterogeneous distributions of binding sites over the cross section of the cellular process. (Left) In the homogeneous model, tau molecules diffuse and can bind at every position in the cellular process. (Middle) If the fine structure of MT packing in the cellular process is explicitly considered, tau molecules diffuse in cytosol and interact with binding sites that are concentrated on MT filaments. The average MT-MT distance (_R_MT-MT) in processes of PC12 cells significantly exceeds the size of each MT filament (_R_MT). (Right) The effective medium approach (EMA) properly homogenizes the heterogeneous model on scales _R_MT << L << R (gray circle), thus implicitly accounting for the fine MT structure, in contrast to a simple assumption that the binding sites are smoothed over the volume of the process (left). To see this figure in color, go online.
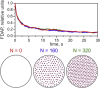
Figure 3
Monte Carlo simulations of FDAP transients for a nonreactive molecule diffusing through the MT network along the cellular process at various densities of MT packing. The FDAP transients were computed as described in the Supporting Material (D = 3 _μ_m2/s, σ = 2 _μ_m). The decays were unaffected by the density of MT packing (i.e., the volume fill factor), which was chosen to be less than the percolation threshold (φ = 0.5) for transversal diffusion (see Eq. 10A). To see this figure in color, go online.
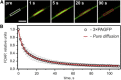
Figure 4
FDAP of 3×PAGFP in cellular processes of PC12 cells. (A) A confocal image series of a PC12 cell transfected to express 3×PAGFP. The photoactivation region (with half-length σ = 8 _μ_m) was located in the middle of cellular processes. Scale bar, 10 _μ_m. (B) The experimental FDAP transient (black circles, mean ± SD, n = 38) was well fitted by the pure-diffusion approximation (red curve; see Table 1), yielding D = 13.9 ± 2.4 _μ_m2/s (estimate ± fit error). To see this figure in color, go online.
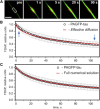
Figure 5
FDAP of PAGFP-tau in processes of PC12 cells. (A) A confocal image series of a PC12 cell transfected to express PAGFP-tau. Photoactivation was performed in the middle of the cellular process (with the half-length σ = 4 _μ_m). Scale bar, 10 _μ_m. (B) The experimental FDAP for PAGFP-tau (black circles, mean ± SD, n = 38) is slowed down by transient interactions of tau molecules with MTs, as expected. In contrast to 3×PAGFP, the experimental FDAP transient for PAGFP-tau is not well fitted by either pure or effective diffusion (see Table 1). Blue arrows indicate discrepancies between the fit and the experimental FDAP transient. (C) The full numerical solution yields a better fit (_k∗_on = 6.2 ± 3.2 s−1, _k_off = 0.13 ± 0.06 s−1, estimate ± fit error) compared to pure and effective diffusion, as ensured by the F-test. The full spectrum of estimates provided by the full numerical solution and the four simplified solutions is given in Table 2. To see this figure in color, go online.
Similar articles
- Molecular mechanisms of Tau binding to microtubules and its role in microtubule dynamics in live cells.
Breuzard G, Hubert P, Nouar R, De Bessa T, Devred F, Barbier P, Sturgis JN, Peyrot V. Breuzard G, et al. J Cell Sci. 2013 Jul 1;126(Pt 13):2810-9. doi: 10.1242/jcs.120832. Epub 2013 May 9. J Cell Sci. 2013. PMID: 23659998 - Tau interaction with microtubules in vivo.
Samsonov A, Yu JZ, Rasenick M, Popov SV. Samsonov A, et al. J Cell Sci. 2004 Dec 1;117(Pt 25):6129-41. doi: 10.1242/jcs.01531. J Cell Sci. 2004. PMID: 15564376 - Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons.
Janning D, Igaev M, Sündermann F, Brühmann J, Beutel O, Heinisch JJ, Bakota L, Piehler J, Junge W, Brandt R. Janning D, et al. Mol Biol Cell. 2014 Nov 5;25(22):3541-51. doi: 10.1091/mbc.E14-06-1099. Epub 2014 Aug 27. Mol Biol Cell. 2014. PMID: 25165145 Free PMC article. - FRET and FRAP imaging: approaches to characterise tau and stathmin interactions with microtubules in cells.
Nouar R, Devred F, Breuzard G, Peyrot V. Nouar R, et al. Biol Cell. 2013 Apr;105(4):149-61. doi: 10.1111/boc.201200060. Epub 2013 Feb 15. Biol Cell. 2013. PMID: 23312015 Review. - Quantitative measurement of intracellular protein dynamics using photobleaching or photoactivation of fluorescent proteins.
Matsuda T, Nagai T. Matsuda T, et al. Microscopy (Oxf). 2014 Dec;63(6):403-8. doi: 10.1093/jmicro/dfu033. Epub 2014 Sep 28. Microscopy (Oxf). 2014. PMID: 25268018 Review.
Cited by
- Presence of a carboxy-terminal pseudorepeat and disease-like pseudohyperphosphorylation critically influence tau's interaction with microtubules in axon-like processes.
Niewidok B, Igaev M, Sündermann F, Janning D, Bakota L, Brandt R. Niewidok B, et al. Mol Biol Cell. 2016 Nov 7;27(22):3537-3549. doi: 10.1091/mbc.E16-06-0402. Epub 2016 Aug 31. Mol Biol Cell. 2016. PMID: 27582388 Free PMC article. - Insights into Disease-Associated Tau Impact on Mitochondria.
Szabo L, Eckert A, Grimm A. Szabo L, et al. Int J Mol Sci. 2020 Sep 1;21(17):6344. doi: 10.3390/ijms21176344. Int J Mol Sci. 2020. PMID: 32882957 Free PMC article. Review. - Quantitative live cell imaging of a tauopathy model enables the identification of a polypharmacological drug candidate that restores physiological microtubule interaction.
Pinzi L, Conze C, Bisi N, Torre GD, Soliman A, Monteiro-Abreu N, Trushina NI, Krusenbaum A, Dolouei MK, Hellwig A, Christodoulou MS, Passarella D, Bakota L, Rastelli G, Brandt R. Pinzi L, et al. Nat Commun. 2024 Feb 23;15(1):1679. doi: 10.1038/s41467-024-45851-6. Nat Commun. 2024. PMID: 38396035 Free PMC article. - Modeling tau transport in the axon initial segment.
Kuznetsov IA, Kuznetsov AV. Kuznetsov IA, et al. Math Biosci. 2020 Nov;329:108468. doi: 10.1016/j.mbs.2020.108468. Epub 2020 Sep 11. Math Biosci. 2020. PMID: 32920097 Free PMC article. - Tau Biology, Tauopathy, Traumatic Brain Injury, and Diagnostic Challenges.
Castellani RJ, Perry G. Castellani RJ, et al. J Alzheimers Dis. 2019;67(2):447-467. doi: 10.3233/JAD-180721. J Alzheimers Dis. 2019. PMID: 30584140 Free PMC article. Review.
References
- Hirokawa N., Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005;6:201–214. - PubMed
- Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol. 1994;6:74–81. - PubMed
- Chen J., Kanai Y., Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous