Stoichiometry and geometry of the CXC chemokine receptor 4 complex with CXC ligand 12: molecular modeling and experimental validation - PubMed (original) (raw)
Stoichiometry and geometry of the CXC chemokine receptor 4 complex with CXC ligand 12: molecular modeling and experimental validation
Irina Kufareva et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Chemokines and their receptors regulate cell migration during development, immune system function, and in inflammatory diseases, making them important therapeutic targets. Nevertheless, the structural basis of receptor:chemokine interaction is poorly understood. Adding to the complexity of the problem is the persistently dimeric behavior of receptors observed in cell-based studies, which in combination with structural and mutagenesis data, suggest several possibilities for receptor:chemokine complex stoichiometry. In this study, a combination of computational, functional, and biophysical approaches was used to elucidate the stoichiometry and geometry of the interaction between the CXC-type chemokine receptor 4 (CXCR4) and its ligand CXCL12. First, relevance and feasibility of a 2:1 stoichiometry hypothesis was probed using functional complementation experiments with multiple pairs of complementary nonfunctional CXCR4 mutants. Next, the importance of dimers of WT CXCR4 was explored using the strategy of dimer dilution, where WT receptor dimerization is disrupted by increasing expression of nonfunctional CXCR4 mutants. The results of these experiments were supportive of a 1:1 stoichiometry, although the latter could not simultaneously reconcile existing structural and mutagenesis data. To resolve the contradiction, cysteine trapping experiments were used to derive residue proximity constraints that enabled construction of a validated 1:1 receptor:chemokine model, consistent with the paradigmatic two-site hypothesis of receptor activation. The observation of a 1:1 stoichiometry is in line with accumulating evidence supporting monomers as minimal functional units of G protein-coupled receptors, and suggests transmission of conformational changes across the dimer interface as the most probable mechanism of altered signaling by receptor heterodimers.
Keywords: GPCR dimerization; chemokine receptor; cysteine trapping; functional complementation; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Molecular models and experimental designs used in the present study. (A) NMR structure of CXCL12 (skin mesh) in complex with the N terminus of CXCR4 (residues M1–K38, ribbon) (39). Chemokine N terminus (green) and N-loop (blue) correspond to the expected interactions in CRS2 and CRS1, respectively. Receptor residues K25–R30 are shown as spheres, labeled, and colored in order from blue to red. CRS1 residue proximities observed in the NMR structure and maintained throughout the docking simulations include the interaction of CXCR4 K25 (blue sphere) with CXCL12 S16, while the subsequent receptor residues up to R30 (red sphere) are directed away from the chemokine N-loop (blue surface) toward the chemokine C-terminal helix; these proximities are shown as thin black lines. (B) A hybrid 2:1 model of the receptor:chemokine interaction accommodates both NMR proximity restraints (black lines) and the mutagenesis data. (C) A hydrid 1:1 model that accommodates NMR proximity restraints (black lines) is inconsistent with mutagenesis and with the two-site interaction hypothesis, because the N terminus of the chemokine invariably points away from the receptor CRS2. (D) A 1:1 model consistent with the two-site interaction hypothesis contradicts NMR proximity restraints, as receptor residues K25–R30 are directed along the chemokine N-loop toward its N terminus. (E–G) Conceptual designs of the functional complementation (E), dimer dilution (F), and cysteine trapping (G) experiments used in this study to probe the receptor:chemokine stoichiometry and geometry hypotheses.
Fig. 2.
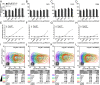
CXCR4 mutants used in this study. (A) Location of mutated residues in the CXCR4 structure. Side view along the membrane plane and top view across the membrane plane from the extracellular side are shown. (B) Surface expression of mutants in HA-tagged and T7-tagged constructs when transiently expressed in CHO-Gα15 cells as determined by flow cytometry analysis of anti-HA and anti-T7 antibody staining. Data are presented as percent of WT receptor expression and represents the average and SD of relative geometric mean fluorescence intensity in at least two independent experiments. (C) Mutant functionality measured as the ability of CHO-Gα15 cells transiently transfected with the mutants to mobilize intracellular Ca2+ in response to stimulation with varying concentrations of CXCL12. Data are presented as percent maximal response elicited by the WT receptor and represents the average and SD of all replicates from at least two independent experiments.
Fig. 3.
CXCR4 mutants retain ability to dimerize with each other (A) and with WT receptor (B and C). In A and B, BRET saturation experiments were performed in HEK293T cells as described in Materials and Methods. The resulting BRETnet ratio is plotted against the fluorescence/luminescence ratio. The BRET pair WT CXCR4-Rluc with CXCR4-YFP was used as a positive control and WT CXCR4-Rluc and GBR2-YFP was used as a negative control for nonspecific BRET. Data are from three independent experiments. In C, HA-tagged mutant receptors were pulled down with Flag-tagged WT receptors using anti-Flag affinity resin when the receptors were coexpressed in HEK293 cells, but not when lysates of two cell populations independently expressing the receptors were mixed (control). The amount of coimmunoprecipitated mutant receptor correlated with the levels of transfection (maximum vs. 50% max HA-tagged mutant).
Fig. 4.
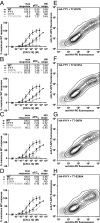
The absence of functional rescue when coexpressing two complementary mutants of CXCR4 in CHO-Gα15 cells. (A–D) CHO-Gα15 cells were transfected with CRS1 mutants, CRS2 mutants, or cotransfected with both and their Ca2+ mobilization measured in response to varying concentrations of CXCL12. For all of the mutant pairs tested, the Ca2+ mobilization response of cotransfected cells did not exceed that of cells transfected with each of the mutants individually. In each experiment, cells transfected with WT CXCR4 were also tested as a positive control. Four representative mutant pairs are shown. Averages and SDs of all replicates in 2–12 independent experiments are shown. (E–H) Mutant coexpression in CHO-Gα15 cells was monitored via flow cytometry by costaining cotransfected cells with PE-conjugated anti-HA antibody and APC-conjugated anti-T7 antibody. Two-dimensional contour plots show that in all cases, the mutants were efficiently coexpressed.
Fig. 5.
Diluting WT-WT dimers by increasing transfection of loss-of-function mutants does not lead to a decrease in signaling. (A–D) Peak fluorescence values from Ca2+ mobilization experiments in which CXCR4 HEK293 tetracycline-inducible cells transfected with the indicated amounts of CRS1 and CRS2 mutants were stimulated with the indicated CXCL12 concentrations. Data for four representative mutants are shown along with averages and SDs of replicates in two to four independent experiments. (E–H) WT and mutant receptor expression levels were monitored by flow cytometry; in all cases, the WT expression was constant and the transfected mutant expression exceeded it two- to sixfold. WT and mutant receptors N-terminally tagged with Flag and HA tags, respectively, were codetected on the cell surface with APC-conjugated anti-Flag antibody and PE-conjugated anti-HA antibody. To normalize geometric mean fluorescence intensity between the two antibodies, a series of samples coexpressing Flag-tagged and HA-tagged WT receptor was costained with these antibodies and also (independently) with anti-CXCR4 antibody (data now shown). (I–L) Coexpression of the two constructs was monitored by flow cytometry.
Fig. 6.
Cysteine trapping experiment with CXCR4 and CXCL12 coexpressed in insect Sf9 cells. (A–C) Nonreducing Western blot analysis of extracts from Sf9 cells coexpressing single Cys mutants of Flag-tagged CXCR4 with single Cys mutants of HA-tagged CXCL12 or its antagonist version, CXCL12(P2G) (15). Molecular weight shift and positive HA-tag staining in the purified material (green circles) indicates spontaneously formed disulfide bond and suggests spatial proximity of the two cysteine residues in the complex, whereas the absence of a chemokine band (red circles) is indicative of spatially distant position of the probed residues. Open circles indicate weak/inconclusive cross-linking. (A) Coexpression samples were probed with HRP-conjugated anti-FLAG and anti-HA antibodies (Top and Bottom, respectively). Flag-CXCR4(K25C) efficiently cross-linked with CXCL12-HA(P2G-S16C) as evidenced by the molecular weight shift and by positive staining with both anti-FLAG and anti-HA antibodies; other probed mutant pairs did not cross-link. (B and C) LI-COR IRDye conjugated secondary antibodies were used to differentially identify Flag-CXCR4 and CXCL12-HA on a single blot. (B) Specificity of the cross-linking reaction in the vicinity of CXCR4 K25 and CXCL12 S16. Flag-CXCR4(K25C) forms strong complexes with CXCL12-HA(S16C) and (E15C), a much weaker complex with CXCL12-HA(H17C) and no complex with CXCL12-HA(V18C). (C) Validation of residue proximities observed in the second-generation 1:1 model of the CXCR4:CXCL12 complex. Flag-CXCR4(F29C) forms a medium strength complex with CXCL12-HA(F13C); Flag-CXCR4(E31C) and (E32C) both form weak complexes with CXCL12-HA(R8C) and (F13C), but not at all with CXCL12-HA(Q59C). (D) Cβ-Cβ distances observed between the probed CXCR4:CXCL12 residue pairs in the NMR structure (39) and second-generation 1:1 complex models. Averages and SDs were calculated using the 20 structures of the NMR ensemble (PDB ID code 2K05) or two top-scoring model conformations. (E and F) Positive and negative cross-links mapped onto 3D structures of CXCR4:CXCL12 complex in the context of the NMR structure (39) (E) or a second-generation 1:1 complex model in Fig. 1_D_ (F). Chemokine orientation is identical between E and F.
Similar articles
- Crosslinking-guided geometry of a complete CXC receptor-chemokine complex and the basis of chemokine subfamily selectivity.
Ngo T, Stephens BS, Gustavsson M, Holden LG, Abagyan R, Handel TM, Kufareva I. Ngo T, et al. PLoS Biol. 2020 Apr 9;18(4):e3000656. doi: 10.1371/journal.pbio.3000656. eCollection 2020 Apr. PLoS Biol. 2020. PMID: 32271748 Free PMC article. - Structural basis for chemokine recognition by a G protein-coupled receptor and implications for receptor activation.
Ziarek JJ, Kleist AB, London N, Raveh B, Montpas N, Bonneterre J, St-Onge G, DiCosmo-Ponticello CJ, Koplinski CA, Roy I, Stephens B, Thelen S, Veldkamp CT, Coffman FD, Cohen MC, Dwinell MB, Thelen M, Peterson FC, Heveker N, Volkman BF. Ziarek JJ, et al. Sci Signal. 2017 Mar 21;10(471):eaah5756. doi: 10.1126/scisignal.aah5756. Sci Signal. 2017. PMID: 28325822 Free PMC article. - Functional anatomy of the full-length CXCR4-CXCL12 complex systematically dissected by quantitative model-guided mutagenesis.
Stephens BS, Ngo T, Kufareva I, Handel TM. Stephens BS, et al. Sci Signal. 2020 Jul 14;13(640):eaay5024. doi: 10.1126/scisignal.aay5024. Sci Signal. 2020. PMID: 32665413 Free PMC article. - Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes in the pathogenesis of bladder cancer.
Nazari A, Khorramdelazad H, Hassanshahi G. Nazari A, et al. Int J Clin Oncol. 2017 Dec;22(6):991-1000. doi: 10.1007/s10147-017-1187-x. Epub 2017 Oct 11. Int J Clin Oncol. 2017. PMID: 29022185 Review. - The good and bad faces of the CXCR4 chemokine receptor.
Teixidó J, Martínez-Moreno M, Díaz-Martínez M, Sevilla-Movilla S. Teixidó J, et al. Int J Biochem Cell Biol. 2018 Feb;95:121-131. doi: 10.1016/j.biocel.2017.12.018. Epub 2017 Dec 27. Int J Biochem Cell Biol. 2018. PMID: 29288743 Review.
Cited by
- Structural basis of antibody inhibition and chemokine activation of the human CC chemokine receptor 8.
Sun D, Sun Y, Janezic E, Zhou T, Johnson M, Azumaya C, Noreng S, Chiu C, Seki A, Arenzana TL, Nicoludis JM, Shi Y, Wang B, Ho H, Joshi P, Tam C, Payandeh J, Comps-Agrar L, Wang J, Rutz S, Koerber JT, Masureel M. Sun D, et al. Nat Commun. 2023 Dec 1;14(1):7940. doi: 10.1038/s41467-023-43601-8. Nat Commun. 2023. PMID: 38040762 Free PMC article. - Heterodimers Are an Integral Component of Chemokine Signaling Repertoire.
Kaffashi K, Dréau D, Nesmelova IV. Kaffashi K, et al. Int J Mol Sci. 2023 Jul 19;24(14):11639. doi: 10.3390/ijms241411639. Int J Mol Sci. 2023. PMID: 37511398 Free PMC article. Review. - Atypical Chemokine Receptor 3 "Senses" CXC Chemokine Receptor 4 Activation Through GPCR Kinase Phosphorylation.
Schafer CT, Chen Q, Tesmer JJG, Handel TM. Schafer CT, et al. Mol Pharmacol. 2023 Oct;104(4):174-186. doi: 10.1124/molpharm.123.000710. Epub 2023 Jul 20. Mol Pharmacol. 2023. PMID: 37474305 Free PMC article. - Computational design of dynamic receptor-peptide signaling complexes applied to chemotaxis.
Jefferson RE, Oggier A, Füglistaler A, Camviel N, Hijazi M, Villarreal AR, Arber C, Barth P. Jefferson RE, et al. Nat Commun. 2023 May 19;14(1):2875. doi: 10.1038/s41467-023-38491-9. Nat Commun. 2023. PMID: 37208363 Free PMC article. - Structure-based discovery of conformationally selective inhibitors of the serotonin transporter.
Singh I, Seth A, Billesbølle CB, Braz J, Rodriguiz RM, Roy K, Bekele B, Craik V, Huang XP, Boytsov D, Pogorelov VM, Lak P, O'Donnell H, Sandtner W, Irwin JJ, Roth BL, Basbaum AI, Wetsel WC, Manglik A, Shoichet BK, Rudnick G. Singh I, et al. Cell. 2023 May 11;186(10):2160-2175.e17. doi: 10.1016/j.cell.2023.04.010. Epub 2023 May 2. Cell. 2023. PMID: 37137306 Free PMC article.
References
- Zou Y-R, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. - PubMed
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–877. - PubMed
- Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11(9):597–606. - PubMed
- Tanegashima K, et al. CXCL14 is a natural inhibitor of the CXCL12-CXCR4 signaling axis. FEBS Lett. 2013;587(12):1731–1735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM081763/GM/NIGMS NIH HHS/United States
- R01 AI37113/AI/NIAID NIH HHS/United States
- R21 AI101687/AI/NIAID NIH HHS/United States
- R01 AI037113/AI/NIAID NIH HHS/United States
- T32 GM007752/GM/NIGMS NIH HHS/United States
- R01 GM071872/GM/NIGMS NIH HHS/United States
- U01 GM094612/GM/NIGMS NIH HHS/United States
- T32 GM008326/GM/NIGMS NIH HHS/United States
- R01 AI118985/AI/NIAID NIH HHS/United States
- U54 GM094618/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources