Protein arginine methyltransferase 5 is a key regulator of the MYCN oncoprotein in neuroblastoma cells - PubMed (original) (raw)
doi: 10.1016/j.molonc.2014.10.015. Epub 2014 Nov 15.
Marianna Szemes 1, Gabriella Cunha Vieira 1, Zsombor Melegh 2, Sally Malik 1, Kate J Heesom 3, Laura Von Wallwitz-Freitas 1, Alexander Greenhough 4, Keith W Brown 1, Y George Zheng 5, Daniel Catchpoole 6, Michael J Deery 7, Karim Malik 8
Affiliations
- PMID: 25475372
- PMCID: PMC4359099
- DOI: 10.1016/j.molonc.2014.10.015
Protein arginine methyltransferase 5 is a key regulator of the MYCN oncoprotein in neuroblastoma cells
Ji Hyun Park et al. Mol Oncol. 2015 Mar.
Abstract
Approximately half of poor prognosis neuroblastomas (NBs) are characterized by pathognomonic MYCN gene amplification and MYCN over-expression. Here we present data showing that short-interfering RNA mediated depletion of the protein arginine methyltransferase 5 (PRMT5) in cell-lines representative of NBs with MYCN gene amplification leads to greatly impaired growth and apoptosis. Growth suppression is not apparent in the MYCN-negative SH-SY5Y NB cell-line, or in two immortalized human fibroblast cell-lines. Immunoblotting of NB cell-lines shows that high PRMT5 expression is strongly associated with MYCN-amplification (P < 0.004, Mann-Whitney U-test) and immunohistochemical analysis of primary NBs reveals that whilst PRMT5 protein is ubiquitously expressed in the cytoplasm of most cells, MYCN-amplified tumours exhibit pronounced nuclear PRMT5 staining. PRMT5 knockdown in MYCN-overexpressing cells, including the SHEP-21N cell-line with inducible MYCN expression leads to a dramatic decrease in MYCN protein and MYCN-associated cell-death in SHEP-21N cells. Quantitative gene expression analysis and cycloheximide chase experiments suggest that PRMT5 regulates MYCN at a post-transcriptional level. Reciprocal co-immunoprecipitation experiments demonstrated that endogenous PRMT5 and MYCN interact in both SK-N-BE(2)C and NGP cell lines. By using liquid chromatography - tandem mass spectrometry (LC-MS/MS) analysis of immunoprecipitated MYCN protein, we identified several potential sites of arginine dimethylation on the MYCN protein. Together our studies implicate PRMT5 in a novel mode of MYCN post-translational regulation and suggest PRMT5 plays a major role in NB tumorigenesis. Small-molecule inhibitors of PRMT5 may therefore represent a novel therapeutic strategy for neuroblastoma and other cancers driven by the MYCN oncogene.
Keywords: Arginine methylation; MYCN; Neuroblastoma; PRMT5.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Figure 1
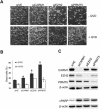
PRMT5 knockdowns induce apoptosis in SK‐N‐BE(2)C cells. (A) Short‐interfering RNAs (siRNAs) targeting CARM1/PRMT4, EZH2 and PRMT5 induced varying degrees of SK‐N‐BE(2)C growth inhibition and cell death after 72 h incubation. The negative control siRNA is also shown (siVE). Cell death was rescuable using QVD. (B) Percentage of dead cells per siRNA treatment are shown without (black bars) and with (white bars) QVD treatment. Significant differences are shown by asterisks (P < 0.05). (C) Verification of knockdowns (upper panel) and apoptosis by immunoblotting for cleaved PARP (cPARP) (lower panel).
Figure 2
PRMT5 knockdown induces apoptosis in the NGP cell‐line, but not in the SH‐SY5Y cell‐line. (A) Cell death triggered by PRMT5 in NGP cells, together with knockdown verification by immunoblotting. No phenotypic changes were observed in SH‐SY5Y cells. (B) Quantification of NGP cell death by cell counting, the asterisk signifying significance (P < 0.05). (C) Confirmation of increased cleaved PARP (cPARP) by immunoblotting following PRMT5 knockdown.
Figure 3
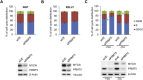
PRMT5 and MYCN protein expression correlations in neuroblastoma. (A) Immunoblotting of PRMT5 in NB cell‐lines without MYCN amplification (MYCN‐un) and cells with amplification (MYCN‐A). Vinculin is used as a loading control. (B) A box plot showing PRMT5 levels normalised to vinculin in cell‐lines demonstrates a significant over‐expression of PRMT5 in MYCN‐A lines (P < 0.004, Mann–Whitney U test). (C) Immunohistochemical staining of NB sections for PRMT5 protein: the top row shows a normal ganglion (g) with cytoplasmic PRMT5 staining arrowed, and Schwannian stroma (ss), followed by a ganglioneuroma with differentiating neuroblasts (d) with cytoplasmic PRMT5 staining arrowed, and finally a differentiating NB, again with cytoplasmic PRMT5. The second row shows differentiating neuroblastomas without MYCN amplification showing predominantly cytoplasmic PRMT5 expression. Neuroblasts (n) and Homer Wright rosettes (hw) are indicated. The third row shows poorly differentiated neuroblastomas with MYCN amplification displaying intense nuclear PRMT5 staining. For PRMT5 immunohistochemistry controls, we used skeletal muscle which is negative for PRMT5 and normal prostate where PRMT5 expression has been reported to be strong in the nucleus of the epithelial cells (Gu et al., 2012) (Figure S3).
Figure 4
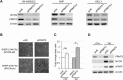
MYCN depletion triggered by PRMT5 knockdown. (A) Immunoblot analysis showing MYCN depletion in SK‐N‐BE(2)C, NGP and Kelly NB cell‐lines following transfection with two independent PRMT5 siRNAs, negating the possibility of off‐target effects. (B) PRMT5 knockdown in the SHEP‐Tet21N cell‐line harbouring inducible MYCN expression. MYCN is switched off in the presence of tetracycline (+Tet) and induced after its removal (−Tet). Cell death is clearly visible in the MYCN‐on cells after PRMT5 knockdown. (C) Cell counts showing significantly increased cell death (asterisked, P < 0.005, Student's t‐test) after PRMT5 knockdown in cells induced to express MYCN. A statistically insignificant change (not significant, ns) is observed in MYCN‐off cells. (D) Immunoblots of SHEP‐Tet21N cells demonstrating that PRMT5 also affects MYCN expressed from the inducible transgene. Depletion is accompanied by an increase in cleaved PARP indicative of apoptosis.
Figure 5
PRMT5 regulates MYCN stability. (A) Cycloheximide (CHX) treated samples analysis following negative control (siVE) or PRMT5 (siPRMT5) knockdown in NGP cells (left) and SHEP‐21N cells (right). (B) Plots of densitometric quantification of MYCN protein stability assays. MYCN levels are normalised relative to β‐actin.
Figure 6
Cell‐cycle changes accompanying PRMT5 knockdowns. Following PRMT5 knockdown, moderate increases in G1 were apparent in NGP and Kelly cell‐lines, and statistically significant G1‐arrest was apparent in uninduced SHEP‐Tet21N cells (asterisked, P < 0.0001, Student's t‐test). In induced SHEP‐Tet21N cells, no G1‐arrest was evident; however a significant G2/M‐phase decrease was apparent (asterisked, P < 0.005). The latter analysis was done with duplicate samples.
Figure 7
Physical interaction between endogenous PRMT5 and MYCN. (A) Co‐immunoprecipitation (IP) of MYCN with anti‐PRMT5 antibody (left) and the reciprocal co‐immunoprecipitation (right) in NGP cells. (B) Co‐immunoprecipitations as above in SK‐N‐BE(2)C cells.
Figure 8
Detection of dimethylated arginine residues in MYCN. (A) LC‐MS/MS MS/MS spectrum of the doubly dimethylated peptide, EPAPVPAAPASAPAAGPAVASGAGIAAPAGAPGVAPPRPGGR (m/z 1211.66, 3+) from MYCN immunoprecipitated after control siRNA knockdown. The majority of ions are due to doubly charged C‐terminal y ions, which show that the two C‐terminal arginine residues are dimethylated (underlined arginines are dimethylated). (B) MS/MS spectrum of the singly dimethylated peptide EPAPVPAAPASAPAAGPAVASGAGIAAPAGAPGVAPPRPGGR (m/z 1202.30, 3+) from MYCN immunoprecipitated after PRMT5 knockdown. The doubly charged C‐terminal fragment ions in this spectrum are 14 m/z units (corresponding to a mass difference of 28 Da) lower than those shown in (A) as a result of the absence of a second dimethylated arginine residue, corresponding to R242 in MYCN (see Figure S5).
Similar articles
- Spliceosomal vulnerability of MYCN-amplified neuroblastoma is contingent on PRMT5-mediated regulation of epitranscriptomic and metabolomic pathways.
Bojko J, Kollareddy M, Szemes M, Bellamy J, Poon E, Moukachar A, Legge D, Vincent EE, Jones N, Malik S, Greenhough A, Paterson A, Park JH, Gallacher K, Chesler L, Malik K. Bojko J, et al. Cancer Lett. 2024 Nov 1;604:217263. doi: 10.1016/j.canlet.2024.217263. Epub 2024 Sep 21. Cancer Lett. 2024. PMID: 39313128 - Expression of a MYCN-interacting isoform of the tumor suppressor BIN1 is reduced in neuroblastomas with unfavorable biological features.
Tajiri T, Liu X, Thompson PM, Tanaka S, Suita S, Zhao H, Maris JM, Prendergast GC, Hogarty MD. Tajiri T, et al. Clin Cancer Res. 2003 Aug 15;9(9):3345-55. Clin Cancer Res. 2003. PMID: 12960121 - Protein arginine methyltransferase 1 is a novel regulator of MYCN in neuroblastoma.
Eberhardt A, Hansen JN, Koster J, Lotta LT Jr, Wang S, Livingstone E, Qian K, Valentijn LJ, Zheng YG, Schor NF, Li X. Eberhardt A, et al. Oncotarget. 2016 Sep 27;7(39):63629-63639. doi: 10.18632/oncotarget.11556. Oncotarget. 2016. PMID: 27571165 Free PMC article. - The MYCN oncoprotein as a drug development target.
Lu X, Pearson A, Lunec J. Lu X, et al. Cancer Lett. 2003 Jul 18;197(1-2):125-30. doi: 10.1016/s0304-3835(03)00096-x. Cancer Lett. 2003. PMID: 12880971 Review. - MYCN oncoprotein targets and their therapeutic potential.
Bell E, Chen L, Liu T, Marshall GM, Lunec J, Tweddle DA. Bell E, et al. Cancer Lett. 2010 Jul 28;293(2):144-57. doi: 10.1016/j.canlet.2010.01.015. Epub 2010 Feb 13. Cancer Lett. 2010. PMID: 20153925 Review.
Cited by
- PRMT5-dependent transcriptional repression of c-Myc target genes promotes gastric cancer progression.
Liu M, Yao B, Gui T, Guo C, Wu X, Li J, Ma L, Deng Y, Xu P, Wang Y, Yang D, Li Q, Zeng X, Li X, Hu R, Ge J, Yu Z, Chen Y, Chen B, Ju J, Zhao Q. Liu M, et al. Theranostics. 2020 Mar 15;10(10):4437-4452. doi: 10.7150/thno.42047. eCollection 2020. Theranostics. 2020. PMID: 32292506 Free PMC article. - Protein arginine methyltransferase 5 (PRMT5) activates WNT/β-catenin signalling in breast cancer cells via epigenetic silencing of DKK1 and DKK3.
Shailesh H, Siveen KS, Sif S. Shailesh H, et al. J Cell Mol Med. 2021 Feb;25(3):1583-1600. doi: 10.1111/jcmm.16260. Epub 2021 Jan 18. J Cell Mol Med. 2021. PMID: 33462997 Free PMC article. - Role of protein arginine methyltransferase 5 in group 3 (MYC-driven) Medulloblastoma.
Chaturvedi NK, Mahapatra S, Kesherwani V, Kling MJ, Shukla M, Ray S, Kanchan R, Perumal N, McGuire TR, Sharp JG, Joshi SS, Coulter DW. Chaturvedi NK, et al. BMC Cancer. 2019 Nov 6;19(1):1056. doi: 10.1186/s12885-019-6291-z. BMC Cancer. 2019. PMID: 31694585 Free PMC article. - Small Molecule Inhibitors of Protein Arginine Methyltransferases.
Hu H, Qian K, Ho MC, Zheng YG. Hu H, et al. Expert Opin Investig Drugs. 2016;25(3):335-58. doi: 10.1517/13543784.2016.1144747. Epub 2016 Feb 16. Expert Opin Investig Drugs. 2016. PMID: 26789238 Free PMC article. Review. - LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/β-catenin signalling in neuroblastoma.
Vieira GC, Chockalingam S, Melegh Z, Greenhough A, Malik S, Szemes M, Park JH, Kaidi A, Zhou L, Catchpoole D, Morgan R, Bates DO, Gabb PD, Malik K. Vieira GC, et al. Oncotarget. 2015 Nov 24;6(37):40053-67. doi: 10.18632/oncotarget.5548. Oncotarget. 2015. PMID: 26517508 Free PMC article.
References
- Brodeur, G.M. , 2003. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer. 3, 203–216. - PubMed
- Chetcuti, A. , Mackie, N. , Tafavogh, S. , Graf, N. , Henwood, T. , Charlton, A. , Catchpoole, D. , 2014. Can archival tissue reveal answers to modern research questions?: computer-aided histological assessment of neuroblastoma tumours collected over 60 years. Microarrays. 2014, (3) 72–88. <10.3390/microarrays3010072> - DOI - PMC - PubMed
- Cho, E.C. , Zheng, S. , Munro, S. , Liu, G. , Carr, S.M. , Moehlenbrink, J. , Lu, Y.C. , Stimson, L. , Khan, O. , Konietzny, R. , McGouran, J. , Coutts, A.S. , Kessler, B. , Kerr, D.J. , Thangue, N.B. , 2012. Arginine methylation controls growth regulation by E2F-1. EMBO J. 31, 1785–1797. - PMC - PubMed
- Chung, J. , Karkhanis, V. , Tae, S. , Yan, F. , Smith, P. , Ayers, L.W. , 2013. Protein arginine methyltransferase 5 inhibition induces lymphoma cell death through reactivation of the retinoblastoma tumor suppressor pathway and polycomb repressor complex 2 silencing. J. Biol. Chem. 288, 35534–35547. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 12743/CRUK_/Cancer Research UK/United Kingdom
- R01 GM086717/GM/NIGMS NIH HHS/United States
- 11975/CRUK_/Cancer Research UK/United Kingdom
- C20791/A12743/Cancer Research UK/United Kingdom
- S10 RR028859/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous