Radical S-adenosylmethionine (SAM) enzymes in cofactor biosynthesis: a treasure trove of complex organic radical rearrangement reactions - PubMed (original) (raw)
Review
Radical S-adenosylmethionine (SAM) enzymes in cofactor biosynthesis: a treasure trove of complex organic radical rearrangement reactions
Angad P Mehta et al. J Biol Chem. 2015.
Abstract
In this minireview, we describe the radical S-adenosylmethionine enzymes involved in the biosynthesis of thiamin, menaquinone, molybdopterin, coenzyme F420, and heme. Our focus is on the remarkably complex organic rearrangements involved, many of which have no precedent in organic or biological chemistry.
Keywords: Biosynthesis; Deazaflavin; Enzyme Mechanism; Heme; Menaquinone; Molybdopterin; Rearrangement; S-Adenosylmethionine (SAM); Thiamin; radical.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
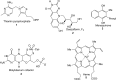
FIGURE 1.
Cofactor biosynthetic pathways make extensive use of radical SAM enzymology. The structures of the five cofactors described in this minireview are shown.
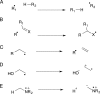
FIGURE 2.
Examples of the major radical reactions found in organic chemistry. A, hydrogen atom abstraction, e.g. 6 to 10 and 14 to 15. B, addition to double bonds, e.g. 15 to 16. C–E, β-bond scission reactions, e.g. 10 to 11, 16 to 17, 17 to 18+19, 19 to 21, 22 to 23, 24 to 25, and 25 to 26. All enzymatic examples are taken from Fig. 3.
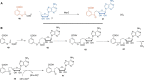
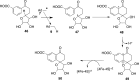
FIGURE 3.
Radical SAM enzymology in thiamin pyrimidine biosynthesis. A, results of a comprehensive labeling study to determine the fate of all of the atoms of AIR (6) during the formation of the HMP-P (8). B, mechanistic proposal for the formation of HMP-P (8).
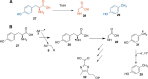
FIGURE 4.
Radical SAM enzymology in thiamin thiazole biosynthesis. A, tyrosine lyase (ThiH)-catalyzed reaction. B, mechanistic proposal for this reaction.
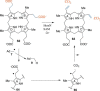
FIGURE 5.
Radical SAM enzymology in deazaflavin biosynthesis. A, F0-synthase-catalyzed reaction. B, mechanistic proposal for F0-synthase.
FIGURE 6.
Radical SAM enzymology in menaquinone biosynthesis. A, reaction catalyzed by aminofutalosine synthase (MqnE). B, mechanistic proposal for the MqnE-catalyzed reaction.
FIGURE 7.
Radical SAM enzymology in menaquinone biosynthesis. Shown is the mechanistic proposal for the MqnC-catalyzed conversion of 46 to 50.
FIGURE 8.
Radical SAM enzymology in molybdopterin biosynthesis. A, MoaA-catalyzed reaction. B, mechanistic proposal for the conversion of GTP (51) to the pterin (52).
FIGURE 9.
Radical SAM enzymology in heme biosynthesis. Shown is the mechanistic proposal for the coproporphyrinogen III oxidase (HemN)-catalyzed formation of protoporphyrinogen IX (63).
Similar articles
- SPASM and twitch domains in S-adenosylmethionine (SAM) radical enzymes.
Grell TA, Goldman PJ, Drennan CL. Grell TA, et al. J Biol Chem. 2015 Feb 13;290(7):3964-71. doi: 10.1074/jbc.R114.581249. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477505 Free PMC article. Review. - Introduction to the thematic minireview series on radical S-adenosylmethionine (SAM) enzymes.
Banerjee R. Banerjee R. J Biol Chem. 2015 Feb 13;290(7):3962-3. doi: 10.1074/jbc.R114.630251. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477525 Free PMC article. Review. - The biosynthesis of thiol- and thioether-containing cofactors and secondary metabolites catalyzed by radical S-adenosylmethionine enzymes.
Jarrett JT. Jarrett JT. J Biol Chem. 2015 Feb 13;290(7):3972-9. doi: 10.1074/jbc.R114.599308. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477512 Free PMC article. Review. - Radical S-adenosyl-L-methionine chemistry in the synthesis of hydrogenase and nitrogenase metal cofactors.
Byer AS, Shepard EM, Peters JW, Broderick JB. Byer AS, et al. J Biol Chem. 2015 Feb 13;290(7):3987-94. doi: 10.1074/jbc.R114.578161. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477518 Free PMC article. Review. - Catalysis of a new ribose carbon-insertion reaction by the molybdenum cofactor biosynthetic enzyme MoaA.
Mehta AP, Hanes JW, Abdelwahed SH, Hilmey DG, Hänzelmann P, Begley TP. Mehta AP, et al. Biochemistry. 2013 Feb 19;52(7):1134-6. doi: 10.1021/bi3016026. Epub 2013 Feb 4. Biochemistry. 2013. PMID: 23286307 Free PMC article.
Cited by
- New Insight into the Mechanism of Anaerobic Heme Degradation.
Mathew LG, Beattie NR, Pritchett C, Lanzilotta WN. Mathew LG, et al. Biochemistry. 2019 Nov 19;58(46):4641-4654. doi: 10.1021/acs.biochem.9b00841. Epub 2019 Nov 7. Biochemistry. 2019. PMID: 31652058 Free PMC article. - Radical SAM Enzymes Involved in Tetrapyrrole Biosynthesis and Insertion.
Layer G, Jahn M, Moser J, Jahn D. Layer G, et al. ACS Bio Med Chem Au. 2022 Feb 16;2(3):196-204. doi: 10.1021/acsbiomedchemau.1c00061. eCollection 2022 Jun 15. ACS Bio Med Chem Au. 2022. PMID: 37101575 Free PMC article. Review. - Recent Advances in Enzymatic Complexity Generation: Cyclization Reactions.
Walsh CT, Tang Y. Walsh CT, et al. Biochemistry. 2018 Jun 5;57(22):3087-3104. doi: 10.1021/acs.biochem.7b01161. Epub 2017 Dec 20. Biochemistry. 2018. PMID: 29236467 Free PMC article. Review. - RaS-RiPPs in Streptococci and the Human Microbiome.
Clark KA, Bushin LB, Seyedsayamdost MR. Clark KA, et al. ACS Bio Med Chem Au. 2022 Aug 17;2(4):328-339. doi: 10.1021/acsbiomedchemau.2c00004. Epub 2022 Mar 21. ACS Bio Med Chem Au. 2022. PMID: 35996476 Free PMC article. Review. - β-NAD as a building block in natural product biosynthesis.
Barra L, Awakawa T, Shirai K, Hu Z, Bashiri G, Abe I. Barra L, et al. Nature. 2021 Dec;600(7890):754-758. doi: 10.1038/s41586-021-04214-7. Epub 2021 Dec 8. Nature. 2021. PMID: 34880494
References
- Sofia H. J., Chen G., Hetzler B. G., Reyes-Spindola J. F., Miller N. E. (2001) Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 29, 1097–1106 - PMC - PubMed
- Atkinson J. K., Ingold K. U. (1993) Cytochrome P450 hydroxylation of hydrocarbons: variation in the rate of oxygen rebound using cyclopropyl radical clocks including two new ultrafast probes. Biochemistry 32, 9209–9214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK044083/DK/NIDDK NIH HHS/United States
- R01 DK067081/DK/NIDDK NIH HHS/United States
- R56 DK044083/DK/NIDDK NIH HHS/United States
- DK44083/DK/NIDDK NIH HHS/United States
- R01 DK044083/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources