First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model - PubMed (original) (raw)
First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model
Eva Kontsekova et al. Alzheimers Res Ther. 2014.
Abstract
Introduction: We have identified structural determinants on tau protein that are essential for pathological tau-tau interaction in Alzheimer's disease (AD). These regulatory domains, revealed by monoclonal antibody DC8E8, represent a novel target for tau-directed therapy. In order to validate this target, we have developed an active vaccine, AADvac1.
Methods: A tau peptide encompassing the epitope revealed by DC8E8 was selected for the development of an active vaccine targeting structural determinants on mis-disordered tau protein that are essential for pathological tau-tau interaction. The efficacy of the vaccine was tested in a transgenic rat model of human tauopathies. Toxicology and safety pharmacology studies were conducted under good laboratory practice conditions in multiple rodent and nonrodent species.
Results: We have administered the tau peptide vaccine to a rat model of AD to investigate whether the vaccine can improve its clinical, histopathological and biochemical AD phenotype. Our results show that vaccination induced a robust protective humoral immune response, with antibodies discriminating between pathological and physiological tau. Active immunotherapy reduced the levels of tau oligomers and the extent of neurofibrillary pathology in the brains of transgenic rats. Strikingly, immunotherapy has reduced AD-type hyperphosphorylation of tau by approximately 95%. Also, the tau peptide vaccine improved the clinical phenotype of transgenic animals. Toxicology and safety pharmacology studies showed an excellent safety and tolerability profile of the AADvac1 vaccine.
Conclusions: Active immunisation targeting crucial domains of Alzheimer tau eliminated tau aggregation and neurofibrillary pathology. Most importantly, the AD type of tau hyperphosphorylation was abolished by vaccination across a wide range of AD phospho-epitopes. Our results demonstrate that active immunisation led to elimination of all major hallmarks of neurofibrillary pathology, which was reflected by a profound improvement in the clinical presentation of transgenic rats. This makes the investigated tau peptide vaccine a highly promising candidate therapeutic for the disease-modifying treatment of AD. The tested vaccine displayed a highly favourable safety profile in preclinical toxicity studies, which opens up the possibility of using it for AD prophylaxis in the future. The vaccine has already entered phase I clinical trial under the name AADvac1.
Trial registration: Current Controlled Trials NCT01850238. Registered 7 May 2013.
Figures
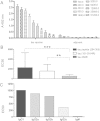
Figure 1
Antibody response in transgenic rats immunised with tau peptide vaccine determined by enzyme-linked immunosorbent assay. Tau peptide vaccine induces high antibody levels specific to the tau peptide 294KDNIKHVPGGGS305. No antibody response was observed in the mice immunised with adjuvant only. Data shown are serial twofold dilutions of animal sera (A). Antibodies elicited by vaccination exhibited statistically significantly higher binding activity to tau peptide and to mis-disordered tau (151-391/4R) than to full-length tau 2N4R (***P = 0.0003 and **P = 0.0028, respectively) (B). EC50, Half-maximal effective concentration. Vaccine-induced antibodies specific to mis-disordered tau are prevalently of the immunoglobulin G (IgG) isotype (C). The data shown are means with error bars representing SD.
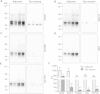
Figure 2
Immunisation with tau peptide vaccine reduced tau oligomers and tau hyperphosphorylation. Western blot analysis with pan-tau monoclonal antibody DC25 showed reduction in oligomeric tau in the brain of transgenic rats treated with tau peptide vaccine (A). The monomeric endogenous rat tau proteins run between 43 and 68 kDa marker bands, whereas monomeric transgenic tau comprises multiple phospho-species between 29 and 43 kDa marker bands. In the vaccine-treated animals, there are only remnants of nonphosphorylated transgene running just above the 29 kDa marker band. Western blot analysis revealed significant reduction of hyperphosphorylated tau species phosphorylated at Thr217 (monoclonal antibody (mAb) DC217) (B), pThr231 (mAb DC209) (C), pSer202/pThr205 (mAb AT8) (D) and pThr181 (mAb DC179) (E). (F) The graph represents the quantification and statistical evaluation of the difference between animals treated with vaccine and those treated with adjuvant only; *P < 0.05, **P < 0.01 . A 6-μl sarkosyl-insoluble fraction was loaded per lane, which corresponds to 30 mg of tissue. Loading of an equal amount of sarkosyl-insoluble proteins, and the efficiency of electroblotting was verified by staining the membrane with Ponceau S (Additional file 1).
Figure 3
Active vaccination reduced the number of transgenic rats developing extensive neurofibrillary pathology. Immunostaining with AT8, pT212 and pS214 shows low numbers of neurofibrillary tangles in the brainstem of treated transgenic rats (A), (D) and (G) compared with untreated transgenic rats (B), (E) and (H). Immunisation lowered the number of transgenic rats with extensive neurofibrillary degeneration by 55% (C) and (F) or by 77% (I).
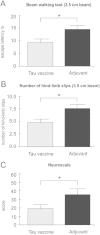
Figure 4
Tau vaccine improves sensorimotor functions of transgenic rats. Tau vaccine–treated transgenic rats showed decreased escape latency (A) and reduced hindlimb slips (B) compared to adjuvant-treated controls. Tau vaccine–treated transgenic rats had significantly lower NeuroScale scores than adjuvant controls, which reflects an improvement of sensorimotor functions (C), *P < 0.05.
Figure 5
Sera from transgenic rats immunised with tau peptide recognised neurofibrillary degeneration in the Alzheimer’s disease brain. Three representative sera obtained from rats immunised with tau peptide recognised neurofibrillary tangles and neuropil threads in the entorhinal cortex of Alzheimer’s disease brain **(A)**-through (C). In contrast, serum from a rat immunised with adjuvant did not recognise any neurofibrillary pathology (D). Scale bar = 100 μm.
Similar articles
- FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease.
Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Zilka N, Winblad B, Novak M. Novak P, et al. Alzheimers Res Ther. 2018 Oct 24;10(1):108. doi: 10.1186/s13195-018-0436-1. Alzheimers Res Ther. 2018. PMID: 30355322 Free PMC article. Clinical Trial. - Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer's disease.
Kontsekova E, Zilka N, Kovacech B, Skrabana R, Novak M. Kontsekova E, et al. Alzheimers Res Ther. 2014 Aug 1;6(4):45. doi: 10.1186/alzrt277. eCollection 2014. Alzheimers Res Ther. 2014. PMID: 25478018 Free PMC article. - Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer's disease: a randomised, double-blind, placebo-controlled, phase 1 trial.
Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, Vince-Kazmerova Z, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Winblad B, Novak M. Novak P, et al. Lancet Neurol. 2017 Feb;16(2):123-134. doi: 10.1016/S1474-4422(16)30331-3. Epub 2016 Dec 10. Lancet Neurol. 2017. PMID: 27955995 Clinical Trial. - AADvac1, an Active Immunotherapy for Alzheimer's Disease and Non Alzheimer Tauopathies: An Overview of Preclinical and Clinical Development.
Novak P, Zilka N, Zilkova M, Kovacech B, Skrabana R, Ondrus M, Fialova L, Kontsekova E, Otto M, Novak M. Novak P, et al. J Prev Alzheimers Dis. 2019;6(1):63-69. doi: 10.14283/jpad.2018.45. J Prev Alzheimers Dis. 2019. PMID: 30569088 Review. - Immunotherapy for Alzheimer's disease: from anti-β-amyloid to tau-based immunization strategies.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, Greco A, Seripa D, Pilotto A. Panza F, et al. Immunotherapy. 2012 Feb;4(2):213-38. doi: 10.2217/imt.11.170. Immunotherapy. 2012. PMID: 22339463 Review.
Cited by
- Experimental Disease-Modifying Agents for Frontotemporal Lobar Degeneration.
Giunta M, Solje E, Gardoni F, Borroni B, Benussi A. Giunta M, et al. J Exp Pharmacol. 2021 Mar 24;13:359-376. doi: 10.2147/JEP.S262352. eCollection 2021. J Exp Pharmacol. 2021. PMID: 33790662 Free PMC article. Review. - High tau levels in cerebrospinal fluid predict nursing home placement and rapid progression in Alzheimer's disease.
Degerman Gunnarsson M, Ingelsson M, Blennow K, Basun H, Lannfelt L, Kilander L. Degerman Gunnarsson M, et al. Alzheimers Res Ther. 2016 Jun 6;8(1):22. doi: 10.1186/s13195-016-0191-0. Alzheimers Res Ther. 2016. PMID: 27263933 Free PMC article. - Disentangling tau: One protein, many therapeutic approaches.
Lane-Donovan C, Boxer AL. Lane-Donovan C, et al. Neurotherapeutics. 2024 Mar;21(2):e00321. doi: 10.1016/j.neurot.2024.e00321. Epub 2024 Jan 25. Neurotherapeutics. 2024. PMID: 38278659 Free PMC article. Review. - FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer's disease.
Novak P, Schmidt R, Kontsekova E, Kovacech B, Smolek T, Katina S, Fialova L, Prcina M, Parrak V, Dal-Bianco P, Brunner M, Staffen W, Rainer M, Ondrus M, Ropele S, Smisek M, Sivak R, Zilka N, Winblad B, Novak M. Novak P, et al. Alzheimers Res Ther. 2018 Oct 24;10(1):108. doi: 10.1186/s13195-018-0436-1. Alzheimers Res Ther. 2018. PMID: 30355322 Free PMC article. Clinical Trial. - Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer's Disease.
Dhakal S, Kushairi N, Phan CW, Adhikari B, Sabaratnam V, Macreadie I. Dhakal S, et al. Int J Mol Sci. 2019 Oct 14;20(20):5090. doi: 10.3390/ijms20205090. Int J Mol Sci. 2019. PMID: 31615073 Free PMC article. Review.
References
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA. et al.Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. - PMC - PubMed
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. e2. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical