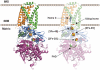Protein-mediated assembly of succinate dehydrogenase and its cofactors - PubMed (original) (raw)
Review
Protein-mediated assembly of succinate dehydrogenase and its cofactors
Jonathan G Van Vranken et al. Crit Rev Biochem Mol Biol. 2015 Mar-Apr.
Abstract
Succinate dehydrogenase (or complex II; SDH) is a heterotetrameric protein complex that links the tribarboxylic acid cycle with the electron transport chain. SDH is composed of four nuclear-encoded subunits that must translocate independently to the mitochondria and assemble into a mature protein complex embedded in the inner mitochondrial membrane. Recently, it has become clear that failure to assemble functional SDH complexes can result in cancer and neurodegenerative syndromes. The effort to thoroughly elucidate the SDH assembly pathway has resulted in the discovery of four subunit-specific assembly factors that aid in the maturation of individual subunits and support the assembly of the intact complex. This review will focus on these assembly factors and assess the contribution of each factor to the assembly of SDH. Finally, we propose a model of the SDH assembly pathway that incorporates all extant data.
Keywords: Assembly factors; redox-active cofactors; respiratory chain; succinate dehydrogenase.
Figures
Figure 1. Porcine succinate dehydrogenase (PDB accession number: 1ZOY) embedded in the mitochondrial inner membrane
SdhA (purple ribbon); SdhB (blue ribbon); SdhC (green ribbon); SdhD (brown ribbon); FAD (green stick); FeS centers, [2Fe-2S], [4Fe-4S], [3Fe-4S] from the bottom (red and yellow sphere); Ubiquinone in the QP site (red stick); Heme b (blue stick)
Figure 2. Iron-sulfur cluster synthesis and delivery to Sdh2
The tan box depicts the de novo synthesis of 2Fe-2S cluster within the ISU complex, in which Isu1 is the scaffold protein. Isu1 interacts with Nfs1 (cysteine desulfurase) and Yah1 (ferredoxin) and receive sulfide ion (S2−) for the synthesis. It is not clear how ferrous ion (Fe2+) is delivered to the ISU complex. The preformed 2Fe-2S cluster is transferred to GSH-bound Grx5 from Isu1 as Ssq1 (Hsp70) and Jac1 (DnaJ protein) are recruited to the ISU complex. The GSH-Grx5 delivers 2Fe-2s clusters to the ISA complex for the subsequent 4Fe-4S cluster synthesis depicted in the blue box. However, it is elusive whether the GSH-Grx5 also is required for 2Fe-2S cluster delivery to the final recipient protein, in this case, Sdh2, or not. It is also unknown whether the preformed 4Fe-4S clusters are directly delivered to the Sdh2 or another factor is in need of this delivery step. All arrows with solid lines indicate transfer of components of Fe-S clusters or pre-formed Fe-S clusters.
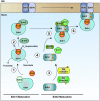
Figure 3. Model of the SDH assembly pathway
Each SDH core subunit is translated in the cytosol and must be subsequently translocated to the mitochondria. Upon mitochondrial import, apo-Sdh1 is rapidly bound by the subunit-specific chaperone, Sdh5, forming a dimeric complex that supports covalent attachment of the FAD cofactor (1). Following covalent flavinylation, the Sdh1-Sdh5 dimer disintegrates resulting in a pool of flavinylated Sdh1 that is unbound by any other core subunits. This leads to the formation of a complex comprised of Sdh1 and Sdh8, another subunit-specific chaperone (2). The formation of this complex supports the formation of the subsequent Sdh1-Sdh2 soluble dimer and also prevents the spurious production of superoxide by flavinylated Sdh1. Meanwhile, apo-Sdh2 must also mature into a complex-competent subunit. This process involves the insertion of 3 Fe-S clusters generated by the ISU and ISA complexes. (3). Following maturation of Sdh2, it interacts with Sdh6 and Sdh7, which serve to protect exposed Fe-S clusters during the assembly process (4) and further associates with a mature Sdh1 subunit forming a heterotetrameric assembly intermediate (5). Finally, the Sdh1-Sdh2 hydrophillic head docks to the IMM via interactions with the Sdh3-Sdh4 membrane anchor domain, which may or may not preassemble at the IMM (6). In the end, the concerted efforts of core subunits, dedicated assembly factors, and other ancillary factors facilitate the stepwise assembly of SDH.
Similar articles
- The assembly of succinate dehydrogenase: a key enzyme in bioenergetics.
Moosavi B, Berry EA, Zhu XL, Yang WC, Yang GF. Moosavi B, et al. Cell Mol Life Sci. 2019 Oct;76(20):4023-4042. doi: 10.1007/s00018-019-03200-7. Epub 2019 Jun 24. Cell Mol Life Sci. 2019. PMID: 31236625 Free PMC article. Review. - SDH mutations in cancer.
Bardella C, Pollard PJ, Tomlinson I. Bardella C, et al. Biochim Biophys Acta. 2011 Nov;1807(11):1432-43. doi: 10.1016/j.bbabio.2011.07.003. Epub 2011 Jul 13. Biochim Biophys Acta. 2011. PMID: 21771581 Review. - Assembly of mitochondrial succinate dehydrogenase in human health and disease.
Cao K, Xu J, Cao W, Wang X, Lv W, Zeng M, Zou X, Liu J, Feng Z. Cao K, et al. Free Radic Biol Med. 2023 Oct;207:247-259. doi: 10.1016/j.freeradbiomed.2023.07.023. Epub 2023 Jul 23. Free Radic Biol Med. 2023. PMID: 37490987 Review. - The Saccharomyces cerevisiae TCM62 gene encodes a chaperone necessary for the assembly of the mitochondrial succinate dehydrogenase (complex II).
Dibrov E, Fu S, Lemire BD. Dibrov E, et al. J Biol Chem. 1998 Nov 27;273(48):32042-8. doi: 10.1074/jbc.273.48.32042. J Biol Chem. 1998. PMID: 9822678 - The conserved RGxxE motif of the bacterial FAD assembly factor SdhE is required for succinate dehydrogenase flavinylation and activity.
McNeil MB, Fineran PC. McNeil MB, et al. Biochemistry. 2013 Oct 29;52(43):7628-40. doi: 10.1021/bi401006a. Epub 2013 Oct 18. Biochemistry. 2013. PMID: 24070374
Cited by
- Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions.
Protasoni M, Zeviani M. Protasoni M, et al. Int J Mol Sci. 2021 Jan 8;22(2):586. doi: 10.3390/ijms22020586. Int J Mol Sci. 2021. PMID: 33435522 Free PMC article. Review. - Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes.
Signes A, Fernandez-Vizarra E. Signes A, et al. Essays Biochem. 2018 Jul 20;62(3):255-270. doi: 10.1042/EBC20170098. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 30030361 Free PMC article. Review. - Accumulation of oncometabolite D-2-Hydroxyglutarate by SLC25A1 inhibition: A metabolic strategy for induction of HR-ness and radiosensitivity.
Xiang K, Kalthoff C, Münch C, Jendrossek V, Matschke J. Xiang K, et al. Cell Death Dis. 2022 Jul 22;13(7):641. doi: 10.1038/s41419-022-05098-9. Cell Death Dis. 2022. PMID: 35869047 Free PMC article. - The assembly of succinate dehydrogenase: a key enzyme in bioenergetics.
Moosavi B, Berry EA, Zhu XL, Yang WC, Yang GF. Moosavi B, et al. Cell Mol Life Sci. 2019 Oct;76(20):4023-4042. doi: 10.1007/s00018-019-03200-7. Epub 2019 Jun 24. Cell Mol Life Sci. 2019. PMID: 31236625 Free PMC article. Review. - The unassembled flavoprotein subunits of human and bacterial complex II have impaired catalytic activity and generate only minor amounts of ROS.
Maklashina E, Rajagukguk S, Iverson TM, Cecchini G. Maklashina E, et al. J Biol Chem. 2018 May 18;293(20):7754-7765. doi: 10.1074/jbc.RA118.001977. Epub 2018 Apr 2. J Biol Chem. 2018. PMID: 29610278 Free PMC article.
References
- AJIT BOLAR N, VANLANDER AV, WILBRECHT C, VAN DER AA N, SMET J, DE PAEPE B, VANDEWEYER G, KOOY F, EYSKENS F, DE LATTER E, DELANGHE G, GOVAERT P, LEROY JG, LOEYS B, LILL R, VAN LAER L, VAN COSTER R. Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum Mol Genet. 2013;22:2590–602. - PubMed
- ALSTON CL, DAVISON JE, MELONI F, VAN DER WESTHUIZEN FH, HE L, HORNIG-DO H-T, PEET AC, GISSEN P, GOFFRINI P, FERRERO I, WASSMER E, MCFARLAND R, TAYLOR RW. Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J Med Gen. 2012;49:569–577. - PMC - PubMed
- BAFUNNO V, GIANCASPERO TA, BRIZIO C, BUFANO D, PASSARELLA S, BOLES E, BARILE M. Riboflavin Uptake and FAD Synthesis in Saccharomyces cerevisiae Mitochondria: INVOLVEMENT OF THE Flx1p CARRIER IN FAD EXPORT. Journal of Biological Chemistry. 2004;279:95–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical