High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease - PubMed (original) (raw)
High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease
Nosratola D Vaziri et al. PLoS One. 2014.
Abstract
Inflammation is a major mediator of CKD progression and is partly driven by altered gut microbiome and intestinal barrier disruption, events which are caused by: urea influx in the intestine resulting in dominance of urease-possessing bacteria; disruption of epithelial barrier by urea-derived ammonia leading to endotoxemia and bacterial translocation; and restriction of potassium-rich fruits and vegetables which are common sources of fermentable fiber. Restriction of these foods leads to depletion of bacteria that convert indigestible carbohydrates to short chain fatty acids which are important nutrients for colonocytes and regulatory T lymphocytes. We hypothesized that a high resistant starch diet attenuates CKD progression. Male Sprague Dawley rats were fed a chow containing 0.7% adenine for 2 weeks to induce CKD. Rats were then fed diets supplemented with amylopectin (low-fiber control) or high fermentable fiber (amylose maize resistant starch, HAM-RS2) for 3 weeks. CKD rats consuming low fiber diet exhibited reduced creatinine clearance, interstitial fibrosis, inflammation, tubular damage, activation of NFkB, upregulation of pro-inflammatory, pro-oxidant, and pro-fibrotic molecules; impaired Nrf2 activity, down-regulation of antioxidant enzymes, and disruption of colonic epithelial tight junction. The high resistant starch diet significantly attenuated these abnormalities. Thus high resistant starch diet retards CKD progression and attenuates oxidative stress and inflammation in rats. Future studies are needed to explore the impact of HAM-RS2 in CKD patients.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
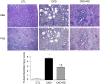
Figure 1. Representative photomicrographs of the PAS & H&E stained kidney sections in a normal control rat, a CKD rat fed low fiber, and a CKD rat fed high resistant starch diet [Magnification x10].
The kidney tissue in the CKD animals exhibited significant tubulo-interstitial injury and fibrosis and heavy inflammatory cell infiltration which were significantly improved with high resistant starch diet (Upper panel). Bar graphs depicting tubulointerstitial injury scores in the study groups (Lower panel).
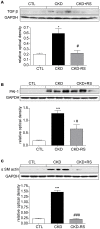
Figure 2. Representative Western blots and group data depicting nuclear content of p65 active subunit of NF-κB and protein abundance of MCP-1, iNOS, COX-1, and COX-2 in the renal tissues of the normal control rats (n = 6) and CKD rats fed low fiber (n = 9) or high resistant starch supplemented diets (n = 9).
Data are means ± SE. *P<0.05, **P<0.01, ***P<0.001 VS CTL group, #P<0.05, ##P<0.01, ###P<0.001 VS CKD group.
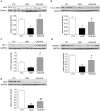
Figure 3. Representative Western blots and group data depicting the NAD(P)H oxidase subunits (NOX-4, gp91phox) and nitrotyrosine abundance in the renal tissues of the normal control rats (n = 6) and CKD rats fed low fiber (n = 9) or high resistant starch supplemented diets (n = 9).
Data are means ± SE. *P<0.05, **P<0.01, ***P<0.001 VS CTL group, #P<0.05, ##P<0.01, ###P<0.001 VS CKD group.
Figure 4. Representative Western blots and group data depicting TGF-β, α-SM actin, and PAI-1 abundance in the renal tissues of the normal control rats (n = 6) and CKD rats fed low fiber (n = 9) or high resistant starch supplemented diets (n = 9).
Data are means ± SE. *P<0.05, **P<0.01, ***P<0.001 VS CTL group, #P<0.05, ##P<0.01, ###P<0.001 VS CKD group.
Figure 5. Representative Western blots and group data depicting nuclear translocation of Nrf2 and protein abundances of its downstream gene products, CuZn-SOD, catalase, heme oxygenase-1 (HO-1) and glutathione peroxidase (GPX) in the renal tissues of the normal control rats (n = 6) and CKD rats fed low fiber (n = 9) or resistant starch supplemented diets (n = 9).
*P<0.05, **P<0.01, ***P<0.001 VS CTL group, #P<0.05, ##P<0.01, ###P<0.001 VS CKD group.
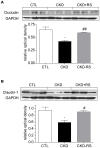
Figure 6. Representative Western blots and group data depicting protein abundances of claudin-1 and occludin in the ascending colons of the normal control rats (n = 6) and CKD rats fed low fiber (n = 9) or resistant starch supplemented diets (n = 9).
Similar articles
- Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: a randomized clinical trial.
Tayebi Khosroshahi H, Vaziri ND, Abedi B, Asl BH, Ghojazadeh M, Jing W, Vatankhah AM. Tayebi Khosroshahi H, et al. Hemodial Int. 2018 Oct;22(4):492-500. doi: 10.1111/hdi.12653. Epub 2018 Mar 13. Hemodial Int. 2018. PMID: 29532981 Clinical Trial. - Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption.
Vaziri ND, Yuan J, Khazaeli M, Masuda Y, Ichii H, Liu S. Vaziri ND, et al. Am J Nephrol. 2013;37(6):518-25. doi: 10.1159/000351171. Epub 2013 May 15. Am J Nephrol. 2013. PMID: 23689670 Free PMC article. - Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats.
Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ, Adams SH. Kieffer DA, et al. Am J Physiol Renal Physiol. 2016 May 1;310(9):F857-71. doi: 10.1152/ajprenal.00513.2015. Epub 2016 Feb 3. Am J Physiol Renal Physiol. 2016. PMID: 26841824 Free PMC article. - Resistant Starch as a Dietary Intervention to Limit the Progression of Diabetic Kidney Disease.
Drake AM, Coughlan MT, Christophersen CT, Snelson M. Drake AM, et al. Nutrients. 2022 Oct 28;14(21):4547. doi: 10.3390/nu14214547. Nutrients. 2022. PMID: 36364808 Free PMC article. Review. - Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment.
Vaziri ND, Zhao YY, Pahl MV. Vaziri ND, et al. Nephrol Dial Transplant. 2016 May;31(5):737-46. doi: 10.1093/ndt/gfv095. Epub 2015 Apr 16. Nephrol Dial Transplant. 2016. PMID: 25883197 Review.
Cited by
- Mizhuo Guanchangye enema delays the decline of renal function in rats with chronic kidney disease by intervening in the TLR4/MyD88/NF-κB pathway.
Li H, Xu P, Zhang X, Ye N, Xu F, Liang B. Li H, et al. Front Med (Lausanne). 2024 Oct 28;11:1454506. doi: 10.3389/fmed.2024.1454506. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39529796 Free PMC article. - Effects of resistant starch supplementation on renal function and inflammatory markers in patients with chronic kidney disease: a meta-analysis of randomized controlled trials.
Zhang Y, Hu XY, Yang SY, Hu YC, Duan K. Zhang Y, et al. Ren Fail. 2024 Dec;46(2):2416609. doi: 10.1080/0886022X.2024.2416609. Epub 2024 Oct 24. Ren Fail. 2024. PMID: 39444299 Free PMC article. - Double-side role of short chain fatty acids on host health via the gut-organ axes.
Gao Y, Yao Q, Meng L, Wang J, Zheng N. Gao Y, et al. Anim Nutr. 2024 May 17;18:322-339. doi: 10.1016/j.aninu.2024.05.001. eCollection 2024 Sep. Anim Nutr. 2024. PMID: 39290857 Free PMC article. Review. - A Historical Perspective on Uremia and Uremic Toxins.
Meijers B, Zadora W, Lowenstein J. Meijers B, et al. Toxins (Basel). 2024 May 15;16(5):227. doi: 10.3390/toxins16050227. Toxins (Basel). 2024. PMID: 38787079 Free PMC article. Review. - Ferroptosis: a potential bridge linking gut microbiota and chronic kidney disease.
Mao ZH, Gao ZX, Pan SK, Liu DW, Liu ZS, Wu P. Mao ZH, et al. Cell Death Discov. 2024 May 15;10(1):234. doi: 10.1038/s41420-024-02000-8. Cell Death Discov. 2024. PMID: 38750055 Free PMC article. Review.
References
- Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S (2013) Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Amer J Nephrol 38:99–103. - PubMed
- Vaziri ND, Wong J, Pahl MV, Piceno YM, Yuan J, et al.. (2013) Chronic kidney disease alters the composition of intestinal microbial flora. Kidney Int 83: 308–15 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was supported in part by intramural USDA-ARS Project 5306-51530-019-00 (SHA) and the Danish Council for Strategic Research (RJM). USDA is an equal opportunity provider and employer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical