Nexus of signaling and endocytosis in oncogenesis driven by non-small cell lung cancer-associated epidermal growth factor receptor mutants - PubMed (original) (raw)
Review
Nexus of signaling and endocytosis in oncogenesis driven by non-small cell lung cancer-associated epidermal growth factor receptor mutants
Byung Min Chung et al. World J Clin Oncol. 2014.
Abstract
Epidermal growth factor receptor (EGFR) controls a wide range of cellular processes, and aberrant EGFR signaling as a result of receptor overexpression and/or mutation occurs in many types of cancer. Tumor cells in non-small cell lung cancer (NSCLC) patients that harbor EGFR kinase domain mutations exhibit oncogene addiction to mutant EGFR, which confers high sensitivity to tyrosine kinase inhibitors (TKIs). As patients invariably develop resistance to TKIs, it is important to delineate the cell biological basis of mutant EGFR-induced cellular transformation since components of these pathways can serve as alternate therapeutic targets to preempt or overcome resistance. NSCLC-associated EGFR mutants are constitutively-active and induce ligand-independent transformation in nonmalignant cell lines. Emerging data suggest that a number of factors are critical for the mutant EGFR-dependent tumorigenicity, and bypassing the effects of TKIs on these pathways promotes drug resistance. For example, activation of downstream pathways such as Akt, Erk, STAT3 and Src is critical for mutant EGFR-mediated biological processes. It is now well-established that the potency and spatiotemporal features of cellular signaling by receptor tyrosine kinases such as EGFR, as well as the specific pathways activated, is determined by the nature of endocytic traffic pathways through which the active receptors traverse. Recent evidence indicates that NSCLC-associated mutant EGFRs exhibit altered endocytic trafficking and they exhibit reduced Cbl ubiquitin ligase-mediated lysosomal downregulation. More recent work has shown that mutant EGFRs undergo ligand-independent traffic into the endocytic recycling compartment, a behavior that plays a key role in Src pathway activation and oncogenesis. These studies are beginning to delineate the close nexus between signaling and endocytic traffic of EGFR mutants as a key driver of oncogenic processes. Therefore, in this review, we will discuss the links between mutant EGFR signaling and endocytic properties, and introduce potential mechanisms by which altered endocytic properties of mutant EGFRs may alter signaling and vice versa as well as their implications for NSCLC therapy.
Keywords: Cbl; Endocytosis; Epidermal growth factor receptor; Non-small cell lung cancer; Signaling; Src; Ubiquitination.
Figures
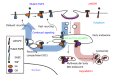
Figure 1
Model of mutant epidermal growth factor receptor endocytic trafficking. Upon ligand binding, activated wtEGFR becomes internalized and localized to endosomes. Internalized EGFR has been linked to Erk and Akt activation. Depending on type of ligand bound, ligand concentration, dimerization partner, mutational statuses and/or availability of other regulators, wtEGFR may recycle to cell surface or be sorted to lysosomes. EGFR bound to EGF is mostly targeted for lysosomes, where it becomes degraded. Sorting of ligand-induced wtEGFR to lysosome is mediated by E3 ubiquitin ligase Cbl which remains attached to receptor throughout endocytosis. Mutant EGFR, however, escapes ligand-induced downregulation through decreased interaction with Cbl, enhanced dimerization with ErbB2, which prefers recycling pathway, and/or constitutive interaction with Src, which antagonizes Cbl. EGFR: Epidermal growth factor receptor.
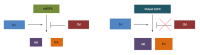
Figure 2
Mutant epidermal growth factor receptor vs wt-epidermal growth factor receptor signaling. While wtEGFR signaling to Akt and Erk is subjected to Cbl-mediated degradation, mutant EGFR cooperates with Src to exaggerate signaling through downstream effectors. EGFR: Epidermal growth factor receptor.
Similar articles
- Aberrant trafficking of NSCLC-associated EGFR mutants through the endocytic recycling pathway promotes interaction with Src.
Chung BM, Raja SM, Clubb RJ, Tu C, George M, Band V, Band H. Chung BM, et al. BMC Cell Biol. 2009 Nov 30;10:84. doi: 10.1186/1471-2121-10-84. BMC Cell Biol. 2009. PMID: 19948031 Free PMC article. - The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants.
Chung BM, Dimri M, George M, Reddi AL, Chen G, Band V, Band H. Chung BM, et al. Oncogene. 2009 Apr 23;28(16):1821-32. doi: 10.1038/onc.2009.31. Epub 2009 Mar 23. Oncogene. 2009. PMID: 19305428 Free PMC article. - Tyrosine kinase inhibitor-induced IL-6/STAT3 activation decreases sensitivity of EGFR-mutant non-small cell lung cancer to icotinib.
Wang J, Wang Y, Zheng C, Hou K, Zhang T, Qu X, Liu Y, Kang J, Hu X, Che X. Wang J, et al. Cell Biol Int. 2018 Sep;42(10):1292-1299. doi: 10.1002/cbin.11000. Epub 2018 Jun 20. Cell Biol Int. 2018. PMID: 29885023 - Mechanisms of resistance to EGFR tyrosine kinase inhibitors.
Huang L, Fu L. Huang L, et al. Acta Pharm Sin B. 2015 Sep;5(5):390-401. doi: 10.1016/j.apsb.2015.07.001. Epub 2015 Jul 26. Acta Pharm Sin B. 2015. PMID: 26579470 Free PMC article. Review.
Cited by
- Increased efficacy of gefitinib on cisplatin-resistant wild-type epidermal growth factor receptor non-small cell lung cancer cells.
Li A, Cao W, Liu X, Zhang Y, Ma Y, Xu R, Tang X. Li A, et al. Transl Cancer Res. 2020 Sep;9(9):5473-5483. doi: 10.21037/tcr-20-1441. Transl Cancer Res. 2020. PMID: 35117912 Free PMC article. - Balancing Safety and Efficacy of Epidermal Growth Factor Receptor Inhibitors in Patients With Squamous Cell Carcinoma of the Head and Neck.
Iberri DJ, Colevas AD. Iberri DJ, et al. Oncologist. 2015 Dec;20(12):1393-403. doi: 10.1634/theoncologist.2015-0177. Epub 2015 Oct 7. Oncologist. 2015. PMID: 26446236 Free PMC article. Review. - AZD9291 overcomes T790 M-mediated resistance through degradation of EGFR(L858R/T790M) in non-small cell lung cancer cells.
Ku BM, Bae YH, Koh J, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Ku BM, et al. Invest New Drugs. 2016 Aug;34(4):407-15. doi: 10.1007/s10637-016-0350-y. Epub 2016 Apr 5. Invest New Drugs. 2016. PMID: 27044261 - SNX5 suppresses clear cell renal cell carcinoma progression by inducing CD44 internalization and epithelial-to-mesenchymal transition.
Zhou Q, Li J, Ge C, Chen J, Tian W, Tian H. Zhou Q, et al. Mol Ther Oncolytics. 2021 Dec 6;24:87-100. doi: 10.1016/j.omto.2021.12.002. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2021. PMID: 35024436 Free PMC article. - Combinatorial high-throughput experimental and bioinformatic approach identifies molecular pathways linked with the sensitivity to anticancer target drugs.
Venkova L, Aliper A, Suntsova M, Kholodenko R, Shepelin D, Borisov N, Malakhova G, Vasilov R, Roumiantsev S, Zhavoronkov A, Buzdin A. Venkova L, et al. Oncotarget. 2015 Sep 29;6(29):27227-38. doi: 10.18632/oncotarget.4507. Oncotarget. 2015. PMID: 26317900 Free PMC article.
References
- Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta. 2006;1766:120–139. - PubMed
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. - PubMed
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. - PubMed
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2011;12:104–117. - PubMed
- Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. - PubMed
Publication types
Grants and funding
- R01 CA087986/CA/NCI NIH HHS/United States
- R01 CA096844/CA/NCI NIH HHS/United States
- R01 CA116552/CA/NCI NIH HHS/United States
- R01 CA144027/CA/NCI NIH HHS/United States
- R01 CA105489/CA/NCI NIH HHS/United States
- R01 CA099163/CA/NCI NIH HHS/United States
- T32 CA009476/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous