Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults - PubMed (original) (raw)
Randomized Controlled Trial
. 2015 Nov;64(11):1744-54.
doi: 10.1136/gutjnl-2014-307913. Epub 2014 Dec 10.
Alexander Viardot 2, Arianna Psichas 1, Douglas J Morrison 3, Kevin G Murphy 4, Sagen E K Zac-Varghese 4, Kenneth MacDougall 5, Tom Preston 3, Catriona Tedford 5, Graham S Finlayson 6, John E Blundell 6, Jimmy D Bell 7, E Louise Thomas 7, Shahrul Mt-Isa 8, Deborah Ashby 8, Glen R Gibson 9, Sofia Kolida 9, Waljit S Dhillo 4, Stephen R Bloom 4, Wayne Morley 10, Stuart Clegg 10, Gary Frost 1
Affiliations
- PMID: 25500202
- PMCID: PMC4680171
- DOI: 10.1136/gutjnl-2014-307913
Randomized Controlled Trial
Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults
Edward S Chambers et al. Gut. 2015 Nov.
Abstract
Objective: The colonic microbiota ferment dietary fibres, producing short chain fatty acids. Recent evidence suggests that the short chain fatty acid propionate may play an important role in appetite regulation. We hypothesised that colonic delivery of propionate would increase peptide YY (PYY) and glucagon like peptide-1 (GLP-1) secretion in humans, and reduce energy intake and weight gain in overweight adults.
Design: To investigate whether propionate promotes PYY and GLP-1 secretion, a primary cultured human colonic cell model was developed. To deliver propionate specifically to the colon, we developed a novel inulin-propionate ester. An acute randomised, controlled cross-over study was used to assess the effects of this inulin-propionate ester on energy intake and plasma PYY and GLP-1 concentrations. The long-term effects of inulin-propionate ester on weight gain were subsequently assessed in a randomised, controlled 24-week study involving 60 overweight adults.
Results: Propionate significantly stimulated the release of PYY and GLP-1 from human colonic cells. Acute ingestion of 10 g inulin-propionate ester significantly increased postprandial plasma PYY and GLP-1 and reduced energy intake. Over 24 weeks, 10 g/day inulin-propionate ester supplementation significantly reduced weight gain, intra-abdominal adipose tissue distribution, intrahepatocellular lipid content and prevented the deterioration in insulin sensitivity observed in the inulin-control group.
Conclusions: These data demonstrate for the first time that increasing colonic propionate prevents weight gain in overweight adult humans.
Trial registration number: NCT00750438.
Keywords: APPETITE; COLONIC FERMENTATION; GUT HORMONES; NUTRITIONAL SUPPLEMENTATION; OBESITY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
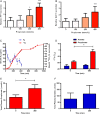
Figure 1
Propionate increases peptide YY (PYY) and glucagon like peptide-1 (GLP-1) release from primary human colonic cells and inulin-propionate ester supplementation delivers propionate to the colon in vivo. Cells isolated from human colonic tissue were incubated with increasing concentrations of propionate. (A) PYY and (B) GLP-1 levels were measured in the supernatants and lysed cells by radioimmunoassay. Percentage gut hormone release per well is expressed relative to the basal release measured (n=4–6). (C) The increase in breath H2 at 240 min suggests that >80% of the labelled propionate entered the colon. (D) Plasma acetate and propionate 13C enrichment (δ13C per mil) at baseline and 360 min. Plasma propionate was significantly more enriched at 360 min whereas no difference was seen in acetate enrichment. Total plasma propionate (E) and acetate (F) concentrations (µmol/L) at baseline and 360 min. Data are presented as mean±SEM, *p<0.05, ***p<0.001.
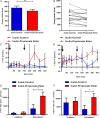
Figure 2
Acute inulin-propionate ester supplementation increases plasma peptide YY (PYY) and glucagon like peptide-1 (GLP-1) levels and reduces energy intake in humans. (A) The mean reduction in energy intake following inulin-control versus inulin-propionate ester. (B) A reduction in energy intake occurred in 16 of the 20 volunteers. (C–F) Plasma gut hormone levels following acute supplementation of inulin-control versus inulin-propionate ester. Arrows indicate standardised meals. Dotted lines signify the time point after which >80% inulin-propionate ester enters the colon as determined by the enrichment of 13C in expired air and breath H2 methodology (figure 1C). Data are presented as mean±SEM, *p<0.05, **p<0.01. AUC, area under the curve.
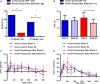
Figure 3
The effect of 24 weeks inulin-control and inulin-propionate ester supplementation on weight gain, liver fat content and gut hormone response. (A) The proportion of subjects who gained 3% or more and 5% or more of their baseline weight at 24 weeks. (B) Intrahepatocellular lipid (IHCL) content at baseline and following 24 weeks of inulin-control and inulin-propionate ester supplementation in subjects with non-alcoholic fatty liver disease (NAFLD). Subjects were identified as having NAFLD on the basis of an IHCL content >5.5% at baseline. Postprandial plasma (C) peptide YY (PYY) and (D) GLP-1 release at baseline and following 24 weeks of inulin-control and inulin-propionate ester supplementation. Data are presented as mean±SEM, *p<0.05.
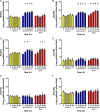
Figure 4
The effect of inulin-propionate ester on the gut microbiota. Bacterial concentrations expressed in Log10 cells/mL culture fluid enumerated using fluorescent in situ hybridisation (FISH) targeting (A) Bifidobacterium spp (Bif164), (B) Bacteroides/Prevotella (Bac303), (C) Atopobium cluster (Ato291), (D) Lactobacillus/Enterococcus (Lab158), (E) Clostridium histolyticum (Chis150) and (F) Eubacterium rectale/Clostridium coccoides (Erec482) at 0 h, 10 h, 24 h, 34 h and 48 h anaerobic, pH controlled faecal batch culture fermentation with control (no substrate), inulin-control and inulin-propionate ester. Data are presented as mean±SEM (n=3), *<0.05, †<0.001, ‡<0.0001 with respect to the 0 h sample.
Similar articles
- Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods.
Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, Tedford C, Fitzpatrick J, Irani C, Busza A, Garcia-Perez I, Fountana S, Holmes E, Goldstone AP, Frost GS. Byrne CS, et al. Am J Clin Nutr. 2016 Jul;104(1):5-14. doi: 10.3945/ajcn.115.126706. Epub 2016 May 11. Am J Clin Nutr. 2016. PMID: 27169834 Free PMC article. Clinical Trial. - The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents.
Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. Psichas A, et al. Int J Obes (Lond). 2015 Mar;39(3):424-9. doi: 10.1038/ijo.2014.153. Epub 2014 Aug 11. Int J Obes (Lond). 2015. PMID: 25109781 Free PMC article. - L-rhamnose as a source of colonic propionate inhibits insulin secretion but does not influence measures of appetite or food intake.
Darzi J, Frost GS, Swann JR, Costabile A, Robertson MD. Darzi J, et al. Appetite. 2016 Mar 1;98:142-9. doi: 10.1016/j.appet.2015.12.011. Epub 2015 Dec 17. Appetite. 2016. PMID: 26706043 Clinical Trial. - Colonic Delivery of Nutrients for Sustained and Prolonged Release of Gut Peptides: A Novel Strategy for Appetite Management.
Kamakura R, Raza GS, Sodum N, Lehto VP, Kovalainen M, Herzig KH. Kamakura R, et al. Mol Nutr Food Res. 2022 Oct;66(19):e2200192. doi: 10.1002/mnfr.202200192. Epub 2022 Aug 19. Mol Nutr Food Res. 2022. PMID: 35938221 Free PMC article. Review. - Appetite-related peptides in childhood and adolescence: role of ghrelin, PYY, and GLP-1.
Horner K, Lee S. Horner K, et al. Appl Physiol Nutr Metab. 2015 Nov;40(11):1089-99. doi: 10.1139/apnm-2015-0050. Epub 2015 Aug 6. Appl Physiol Nutr Metab. 2015. PMID: 26466085 Review.
Cited by
- Clostridium Butyricum CGMCC0313.1 Modulates Lipid Profile, Insulin Resistance and Colon Homeostasis in Obese Mice.
Shang H, Sun J, Chen YQ. Shang H, et al. PLoS One. 2016 Apr 28;11(4):e0154373. doi: 10.1371/journal.pone.0154373. eCollection 2016. PLoS One. 2016. PMID: 27123997 Free PMC article. - Increase in colonic PRopionate as a method of prEVENTing weight gain over 12 months in adults aged 20-40 years (iPREVENT): a multi-centre, double-blind, randomised, parallel-group trial.
Pugh JE, Petropoulou K, Vasconcelos JC, Anjum A, Thom G, McCombie L, Tashkova M, Alshehhi S, Babalis D, Holroyd L, Sadiq BA, Prechtl C, Preston T, Chambers E, Lean MJ, Dhillo W, Prevost AT, Morrison D, Frost G. Pugh JE, et al. EClinicalMedicine. 2024 Sep 25;76:102844. doi: 10.1016/j.eclinm.2024.102844. eCollection 2024 Oct. EClinicalMedicine. 2024. PMID: 39391015 Free PMC article. - Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development.
Kim S, Shin YC, Kim TY, Kim Y, Lee YS, Lee SH, Kim MN, O E, Kim KS, Kweon MN. Kim S, et al. Gut Microbes. 2021 Jan-Dec;13(1):1-20. doi: 10.1080/19490976.2021.1892441. Gut Microbes. 2021. PMID: 33678130 Free PMC article. - The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease.
Chakaroun RM, Olsson LM, Bäckhed F. Chakaroun RM, et al. Nat Rev Cardiol. 2023 Apr;20(4):217-235. doi: 10.1038/s41569-022-00771-0. Epub 2022 Oct 14. Nat Rev Cardiol. 2023. PMID: 36241728 Review. - The Implication of Mechanistic Approaches and the Role of the Microbiome in Polycystic Ovary Syndrome (PCOS): A Review.
Mukherjee AG, Wanjari UR, Kannampuzha S, Murali R, Namachivayam A, Ganesan R, Dey A, Babu A, Renu K, Vellingiri B, Ramanathan G, Priya Doss C G, Elsherbiny N, Elsherbini AM, Alsamman AM, Zayed H, Gopalakrishnan AV. Mukherjee AG, et al. Metabolites. 2023 Jan 14;13(1):129. doi: 10.3390/metabo13010129. Metabolites. 2023. PMID: 36677054 Free PMC article. Review.
References
- Hussain SS, Bloom SR. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes (Lond) 2013;37:625–33. - PubMed
- Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol 2013;11:639–47. - PubMed
- Cani PD. Metabolism in 2013: the gut microbiota manages host metabolism. Nat Rev Endocrinol 2014;10:74–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U120061305/MRC_/Medical Research Council/United Kingdom
- BB/H004815/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- CDF-2009-02-05/DH_/Department of Health/United Kingdom
- BB/H005072/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/L004259/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H004971/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical