n-3 PUFA supplementation benefits microglial responses to myelin pathology - PubMed (original) (raw)
n-3 PUFA supplementation benefits microglial responses to myelin pathology
Songela Chen et al. Sci Rep. 2014.
Abstract
Microglia represent rational but challenging targets for improving white matter integrity because of their dualistic protective and toxic roles. The present study examines the effect of Omega-3 polyunsaturated fatty acids (n-3 PUFAs) on microglial responses to myelin pathology in primary cultures and in the cuprizone mouse model of multiple sclerosis (MS), a devastating demyelination disease. Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), the two main forms of n-3 PUFAs in the brain, inhibited the release of nitric oxide and tumor necrosis factor-α from primary microglia upon IFN-γ and myelin stimulation. DHA and EPA also enhanced myelin phagocytosis in vitro. Therefore, n-3 PUFAs can inhibit inflammation while at the same time enhancing beneficial immune responses such as microglial phagocytosis. In vivo studies demonstrated that n-3 PUFA supplementation reduced cuprizone-induced demyelination and improved motor and cognitive function. The positive effects of n-3 PUFAs were accompanied by a shift in microglial polarization toward the beneficial M2 phenotype both in vitro and in vivo. These results suggest that n-3 PUFAs may be clinically useful as immunomodulatory agents for demyelinating diseases through a novel mechanism involving microglial phenotype switching.
Figures
Figure 1. DHA and EPA inhibit inflammatory responses in primary microglia.
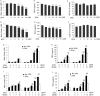
(A–F) Primary microglia seeded at 5 × 104/well were pretreated with varying concentrations of DHA or EPA (5–80 μM) for 24 hrs followed by LPS (2.5 ng/mL) stimulation. Culture medium was collected at 24 hrs after LPS. The production of nitric oxide (NO) (A, D) and tumor necrosis factor-α (TNF-α) (B, E) was measured as markers of inflammation. Production of lactate dehydrogenase (LDH) (C, F) was measured as a cell death assay. (G–J) Primary microglia seeded at 5 × 104/well were pretreated with DHA (20 μM) or EPA (20 μM) for 24 hrs followed by myelin (1, 5 or 10 μg/mL) without or with IFN-γ (5 ng/mL) stimulation for an additional 24 hrs. Extracellular NO (G, I) and TNF-α (H, J) were measured in the culture medium. Results are expressed as mean ± SEM from three to four independent experiments, each performed in triplicate. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus vehicle treatment.
Figure 2. DHA and EPA enhance myelin phagocytosis in primary microglia.
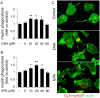
Primary microglia were pretreated with varying concentrations of DHA or EPA (5–80 μM) for 24 hrs, followed by incubation with Cy3-labeled myelin (0.5 μg/mL) for an additional 6 hrs. (A, B) Myelin phagocytosis was quantified as intracellular fluorescence. (C) Representative images showing that DHA (20 μM) or EPA (20 μM) treatment increased myelin phagocytosis. Results are expressed as mean ± SEM from three to four independent experiments, each performed in triplicate. *P ≤ 0.05, **P ≤ 0.01 versus vehicle treatment.
Figure 3. DHA and EPA induce microglial polarization toward the M2 phenotype.
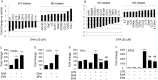
Primary microglia seeded at 5 × 104/well in a 6-well plate were pretreated with DHA or EPA (20 μM each) for 24 hrs. (A–B) Real-time PCR arrays show that DHA (A) and EPA (B) treatment significantly inhibited expression of multiple M1 genes and enhanced expression of M2 genes. (C–F) Real-time PCR showed significant stimulation of M2-related gene CD206 (C) and TGFβ (D) after DHA or EPA treatment. In addition, DHA or EPA pretreatment significantly inhibited the expression of M1-related genes TNF-α (E) and iNOS (F) following LPS (2.5 ng/mL) stimulation for 24 hrs. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus control; ##P ≤ 0.01, ###P ≤ 0.001 versus LPS.
Figure 4. n-3 PUFA supplementation reduces demyelination in cuprizone model of MS.
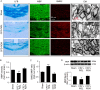
Mice were fed for 5 weeks with a diet containing 0.2% cuprizone with (N3H) or without (N3L) n-3 PUFA enrichment. (A) Demyelinating lesions in the corpus callosum (CC) were revealed by Luxol fast blue (LFB) staining (left), MBP staining, and electron microscopy (EM, right). Pathological changes in axons were revealed by SMI32 immunostaining. Images are representative of sections from four animals. (B) MBP intensity was measured and calculated as fold change over sham. (C) The ratio of SMI32 to MBP was calculated as an indicator of white matter injury (axonal damage and demyelination). (D) Western blot analysis of MBP expression. n = 4 mice for each group. *P ≤ 0.05, **P ≤ 0.01 versus sham controls.
Figure 5. n-3 PUFA supplementation reduces neurological deficits in cuprizone model of demyelination.
Mice were fed for 5 weeks with a diet containing 0.2% cuprizone with (N3H) or without (N3L) n-3 PUFA enrichment. (A–C) The Morris water maze test performed 28–32 days after initiation of the cuprizone diet. (A) Representative images of the swim paths of mice in each group while the platform was present (place navigation; learning phase) and after it was removed (probe test; memory phase). (B) Latency to locate the submerged platform was measured in n-3 PUFA-supplemented and regular diet mice 28–31 days after initiation of the cuprizone diet. (C) Spatial memory of the location of previously submerged platform was measured in n-3 PUFA-supplemented and regular diet mice 32 days after initiation of the cuprizone diet. Spatial memory is expressed as the time spent in the goal quadrant when the platform was removed. (D) Rotarod test before and after 2–5 weeks of cuprizone treatment. n = 8 animals/group. *P ≤ 0.05, **P ≤ 0.01 for indicated comparisons.
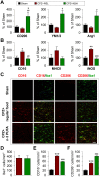
Figure 6. n-3 PUFA supplementation promotes M2 microglial polarization in cuprizone model of demyelination.
Mice were fed for 5 weeks with a diet containing 0.2% cuprizone with (N3H) or without (N3L) n-3 PUFA enrichment. (A–B) Real time-PCR for M1 markers (A) and M2 markers (B) was performed using total RNA extracted from the corpus callosum. (C) Representative double staining immunofluorescence of Iba1 with CD16/32 (M1) or CD206 (M2) in the corpus callosum. Scale bar: 40 μm. (D–F) Quantification of the Iba1+ (D), CD16/32+ (E), and CD206+ (F) cells in the corpus callosum. n = 4 animals per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus sham; #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 versus CPZ + N3L.
Similar articles
- A Post-stroke Therapeutic Regimen with Omega-3 Polyunsaturated Fatty Acids that Promotes White Matter Integrity and Beneficial Microglial Responses after Cerebral Ischemia.
Jiang X, Pu H, Hu X, Wei Z, Hong D, Zhang W, Gao Y, Chen J, Shi Y. Jiang X, et al. Transl Stroke Res. 2016 Dec;7(6):548-561. doi: 10.1007/s12975-016-0502-6. Epub 2016 Oct 7. Transl Stroke Res. 2016. PMID: 27714669 Free PMC article. - Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-β42 by human microglia and decrease inflammatory markers.
Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H, Eriksdotter M, Schultzberg M. Hjorth E, et al. J Alzheimers Dis. 2013;35(4):697-713. doi: 10.3233/JAD-130131. J Alzheimers Dis. 2013. PMID: 23481688 - Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid.
Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Siriwardhana N, et al. Adv Food Nutr Res. 2012;65:211-22. doi: 10.1016/B978-0-12-416003-3.00013-5. Adv Food Nutr Res. 2012. PMID: 22361189 Review. - Neuronal injury in chronic CNS inflammation.
Zindler E, Zipp F. Zindler E, et al. Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
Cited by
- Involvement of Microglia in Neurodegenerative Diseases: Beneficial Effects of Docosahexahenoic Acid (DHA) Supplied by Food or Combined with Nanoparticles.
Charrière K, Ghzaiel I, Lizard G, Vejux A. Charrière K, et al. Int J Mol Sci. 2021 Sep 30;22(19):10639. doi: 10.3390/ijms221910639. Int J Mol Sci. 2021. PMID: 34638979 Free PMC article. Review. - Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases.
Song GJ, Suk K. Song GJ, et al. Front Aging Neurosci. 2017 May 15;9:139. doi: 10.3389/fnagi.2017.00139. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28555105 Free PMC article. Review. - Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers.
Malau IA, Chang JP, Lin YW, Chang CC, Chiu WC, Su KP. Malau IA, et al. Cells. 2024 Oct 29;13(21):1791. doi: 10.3390/cells13211791. Cells. 2024. PMID: 39513898 Free PMC article. Review. - Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases.
Khotimchenko YS, Silachev DN, Katanaev VL. Khotimchenko YS, et al. Mar Drugs. 2022 Nov 11;20(11):708. doi: 10.3390/md20110708. Mar Drugs. 2022. PMID: 36421986 Free PMC article. Review. - The evolving role of neuro-immune interaction in brain repair after cerebral ischemic stroke.
Wang X, Xuan W, Zhu ZY, Li Y, Zhu H, Zhu L, Fu DY, Yang LQ, Li PY, Yu WF. Wang X, et al. CNS Neurosci Ther. 2018 Dec;24(12):1100-1114. doi: 10.1111/cns.13077. Epub 2018 Oct 22. CNS Neurosci Ther. 2018. PMID: 30350341 Free PMC article. Review.
References
- Kutzelnigg A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005). - PubMed
- Kotter M. R., Zhao C., van Rooijen N. & Franklin R. J. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiology of disease 18, 166–175 (2005). - PubMed
- Napoli I. & Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol 225, 24–28 (2010). - PubMed
- Boven L. A. et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials