Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine? - PubMed (original) (raw)
Review
Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine?
Arian Aryani et al. Mol Neurobiol. 2016 Mar.
Abstract
Since the beginning of the last decade, exosomes have been of increased interest in the science community. Exosomes represent a new kind of long distance transfer of biological molecules among cells. This review provides a comprehensive overview about the construction of exosomes, their targeting and their fusion mechanisms to the recipient cells. Complementarily, the current state of research regarding the cargo of exosomes is discussed. A particular focus was placed on the role of exosomes in the central nervous system. An increasing number of physiological processes in the brain could be associated with exosomes. In this context, it is becoming more apparent that exosomes are involved in several neurological and specifically neurodegenerative diseases. The treatment of these kinds of diseases is often difficult not least because of the blood-brain barrier. Exosomes are very stable, can pass the blood-brain barrier and, therefore, reveal bright perspectives towards diagnosis and therapeutic treatments. A prerequisite for clinical applications is a standardised approach. Features necessary for a standardised diagnosis using exosomes are discussed. In therapeutic terms, exosomes represent a promising drug delivery system able to pass the blood-brain barrier. One option to overcome the disadvantages potentially associated with the use of endogenous exosomes is the design of artificial exosomes. The artificial exosomes with a clearly defined therapeutic active cargo and surface marker ensuring the specific targeting to the recipient cells is proposed as a promising approach.
Keywords: Artificial exosome; Diagnosis; Extracellular vesicles; MicroRNA; Multiple sclerosis; Neurodegenerative disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
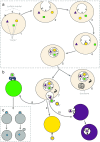
Fig. 1
Formation and release of exosomes. a Exosome formation initiates by inward budding of plasma membrane to form MVBs. By formation of MVBs, receptors on the surface of plasma membrane locate inside the MVBs (1_–_3). Inward budding of MVBs results in the formation of internal vesicles inside the MVBs (4_–_5). By this stage, internal vesicles carry components from inside the cell such as cytosolic proteins or RNAs. Designated targeting molecules such as receptors are located on the outer surface of these vesicles similar to their location on the plasma membrane of the cell. b There are two possible destinations for the MVBs, which contain the internal vesicles. Either they are digested by lysosomes (6) or the MVB membrane fuses with the plasma membrane to release the internal vesicles now called exosomes (7). After reaching to their target cell, exosomes deliver their cargo either by adhesion via members of integrin family (8), via receptor ligand interaction (9) and/or via internalisation by endocytosis (10). c Exosomes deliver their cargo to the target cell either by fusion (left part) or hemifusion (right part). Fusion: the membrane of exosome and the target cell merge and result in an interconnected structure. Hemifusion: after releasing of cargo, the exosome membrane disconnected again from the plasma membrane of the target cell. MVB multivesicular body
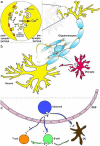
Fig. 2
Active role of exosomes in the central nervous system. a Exosomes play a crucial role in synaptic plasticity. Syt4 is a membrane-trafficking protein and is essential for retrograde signalling. It was observed that exosome containing Syt4 (demonstrated by brown circles, indicated with dark arrows heads) are released from presynaptic terminal and thereby the entire pool of Syt4 in postsynaptic terminal is provided by exosomes from the presynaptic terminal. The yellow arrow presents the release of neurotransmitters (yellow triple-circle shapes) from presynaptic terminal and binding of them to neurotransmitter receptors (orange two-circle shapes). Release of neurotransmitters allows entry of Ca2+ to the presynaptic terminal and activates fusion of exosome-containing MVBs to the plasma membrane. b In the CNS, different cell types such as neurons, oligodendrocytes and microglia release exosomes (exosomes of each cell type are performed by small circles coloured in parental cell colour). Released exosomes reach different cell type in distance and deliver their cargo to other cells. Therefore, cells in the CNS can communicate with each other and help to regulate their function. c Exosomes derived from resident cells in the CNS can pass through the BBB. Mast cell-derived exosomes (dark blue circles) can pass through the BBB and are able to activate B and T cells or induces DCs to become efficient antigen-presenting cells. B cell-derived exosomes, in turn, can stimulate T cells or transfer MHC class II proteins to the surface of follicular DCs, which do not express these molecules
Similar articles
- Exosome-based platforms for treatment of multiple sclerosis.
Zonouz AM, Rahbardar MG, Alibolandi M. Zonouz AM, et al. Brain Res Bull. 2025 Mar;222:111256. doi: 10.1016/j.brainresbull.2025.111256. Epub 2025 Feb 12. Brain Res Bull. 2025. PMID: 39952444 Review. - Exosome-based drug delivery systems for enhanced neurological therapeutics.
Vahab SA, V VK, Kumar VS. Vahab SA, et al. Drug Deliv Transl Res. 2025 Apr;15(4):1121-1138. doi: 10.1007/s13346-024-01710-x. Epub 2024 Sep 26. Drug Deliv Transl Res. 2025. PMID: 39325272 Review. - Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers.
Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, Mandal M. Das CK, et al. Mol Pharm. 2019 Jan 7;16(1):24-40. doi: 10.1021/acs.molpharmaceut.8b00901. Epub 2018 Dec 18. Mol Pharm. 2019. PMID: 30513203 Review. - Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases.
Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Furtado D, et al. Adv Mater. 2018 Nov;30(46):e1801362. doi: 10.1002/adma.201801362. Epub 2018 Jul 31. Adv Mater. 2018. PMID: 30066406 Review. - Exosomes in Parkinson's Disease: Current Perspectives and Future Challenges.
Yuan L, Li JY. Yuan L, et al. ACS Chem Neurosci. 2019 Feb 20;10(2):964-972. doi: 10.1021/acschemneuro.8b00469. Epub 2019 Feb 1. ACS Chem Neurosci. 2019. PMID: 30664350 Review.
Cited by
- Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos.
Alonso-Alonso ML, García-Posadas L, Diebold Y. Alonso-Alonso ML, et al. Stem Cell Rev Rep. 2022 Mar;18(3):854-901. doi: 10.1007/s12015-021-10155-5. Epub 2021 Apr 26. Stem Cell Rev Rep. 2022. PMID: 33904115 Free PMC article. Review. - A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells.
Jurj A, Zanoaga O, Braicu C, Lazar V, Tomuleasa C, Irimie A, Berindan-Neagoe I. Jurj A, et al. Cancers (Basel). 2020 Jan 27;12(2):298. doi: 10.3390/cancers12020298. Cancers (Basel). 2020. PMID: 32012717 Free PMC article. Review. - Potential Role of Extracellular Vesicles in the Pathophysiology of Drug Addiction.
Rao PSS, O'Connell K, Finnerty TK. Rao PSS, et al. Mol Neurobiol. 2018 Aug;55(8):6906-6913. doi: 10.1007/s12035-018-0912-4. Epub 2018 Jan 23. Mol Neurobiol. 2018. PMID: 29363042 Review. - Mesenchymal Stromal Cell-Derived Tailored Exosomes Treat Bacteria-Associated Diabetes Foot Ulcers: A Customized Approach From Bench to Bed.
Raghav A, Tripathi P, Mishra BK, Jeong GB, Banday S, Gautam KA, Mateen QN, Singh P, Singh M, Singla A, Ahmad J. Raghav A, et al. Front Microbiol. 2021 Jul 27;12:712588. doi: 10.3389/fmicb.2021.712588. eCollection 2021. Front Microbiol. 2021. PMID: 34385994 Free PMC article. Review. - Exosomes as immunomodulators in autoimmune inflammation: implications for primary Sjögren's disease.
Hong Y, Chen S, Jiang X, Zhang J, Liang X, Yao J, Gao S, Hua C. Hong Y, et al. Inflamm Res. 2025 Jun 13;74(1):91. doi: 10.1007/s00011-025-02053-0. Inflamm Res. 2025. PMID: 40512213 Review.
References
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10(2):211–219. doi: 10.1038/ncb1682. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources