Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus - PubMed (original) (raw)
Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus
Melissa A Cunningham et al. J Neuroinflammation. 2014.
Abstract
Background: One of the more profound features of systemic lupus erythematosus (SLE) is that females have a 9:1 prevalence of this disease over males. Up to 80% of SLE patients have cognitive defects or affective disorders. The mechanism of CNS injury responsible for cognitive impairment is unknown. We previously showed that ERα deficiency significantly reduced renal disease and increased survival in lupus-prone mice. We hypothesized that ERα deficiency would be similarly protective in the brain, and that ERα may play a role in modulating blood-brain barrier (BBB) integrity and/or neuroinflammation in lupus-prone mice.
Methods: MRL/lpr ERα+/+ and ERαKO mice (n = 46) were ovariectomized, received 17β-estradiol pellets, and underwent radial arm water maze (WRAM) and novel object recognition (NOR) testing starting at eight weeks of age. Mice were sacrificed and brains were hemisected and processed for either immunohistochemistry, or hippocampus and parietal cortex dissection for Western blotting.
Results: MRL/lpr ERαKO mice (n = 21) performed significantly better in WRAM testing than wild-type MRL/lpr mice (n = 25). There was a significant reduction in reference memory errors (P <0.007), working memory errors (P <0.05), and start arm errors (P <0.02) in ERαKO mice. There were significant differences in NOR testing, particularly total exploration time, with ERα deficiency normalizing behavior. No significant differences were seen in markers of tight junction, astrogliosis, or microgliosis in the hippocampus or cortex by Western blot, however, there was a significant reduction in numbers of Iba1+ activated microglia in the hippocampus of ERαKO mice, as evidenced by immunohistochemietry (IHC).
Conclusion: ERα deficiency provides significant protection against cognitive deficits in MRL/lpr mice as early as eight weeks of age. Additionally, the significant reduction in Iba1+ activated microglia in the MRL/lpr ERαKO mice was consistent with reduced inflammation, and may represent a biological mechanism for the cognitive improvement observed.
Figures
Figure 1
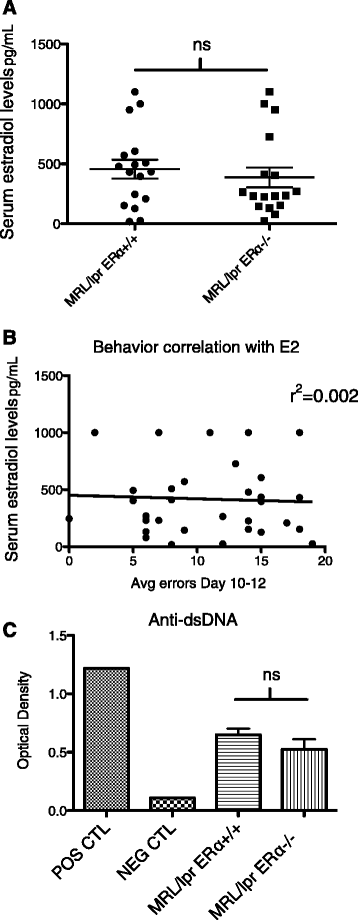
Hormone and autoantibody levels. (A) There was no significant difference in average 17β-estradiol level between the MRL/lpr WT and MRL/lpr ERαKO groups of mice. (B) There was also no correlation between individual serum estradiol levels and behavior (performance in the WRAM). (C) There was no significant difference between MRL/lpr WT and ERαKO mice with regard to anti-dsDNA levels at 10 weeks of age. POS = single sample from a sick/aged MRL/lpr WT; NEG = single sample from a B6 mouse.
Figure 2
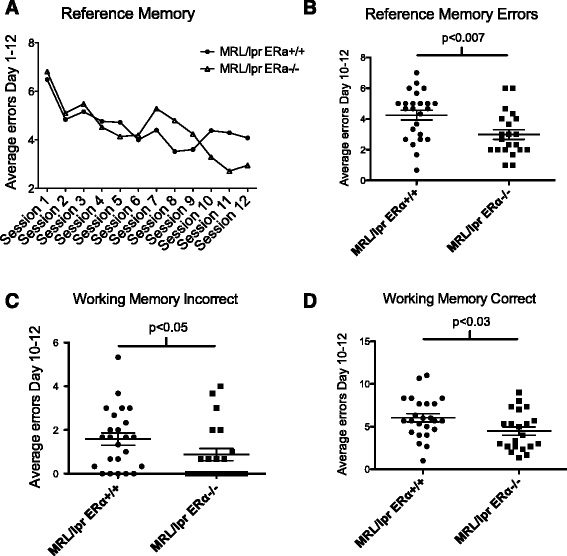
MRL/lpr ERαKO mice perform better than MRL/lpr WT in WRAM testing. (A) Reference memory learning curve. The one-within (session) repeated measures revealed a significant effect of sessions for days 1 to 12. (B) In the late or asymptotic phase (days 10 to 12), MRL/lpr ERαKO mice outperformed MRL/lpr mice, with significantly fewer reference memory errors made. (C, D) Average errors made in the late or asymptotic phase (days 10 to 12) revealed that MRL/lpr ERαKO mice made significantly fewer errors than MRL/lpr mice in both working memory incorrect and working memory correct errors.
Figure 3
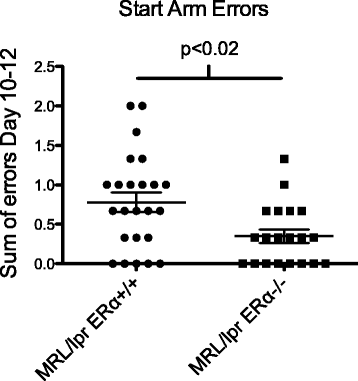
WRAM start arm errors. MRL/lpr mice exhibit abnormal behavior. ERαKO mice made significantly fewer errors versus MRL/lpr mice.
Figure 4
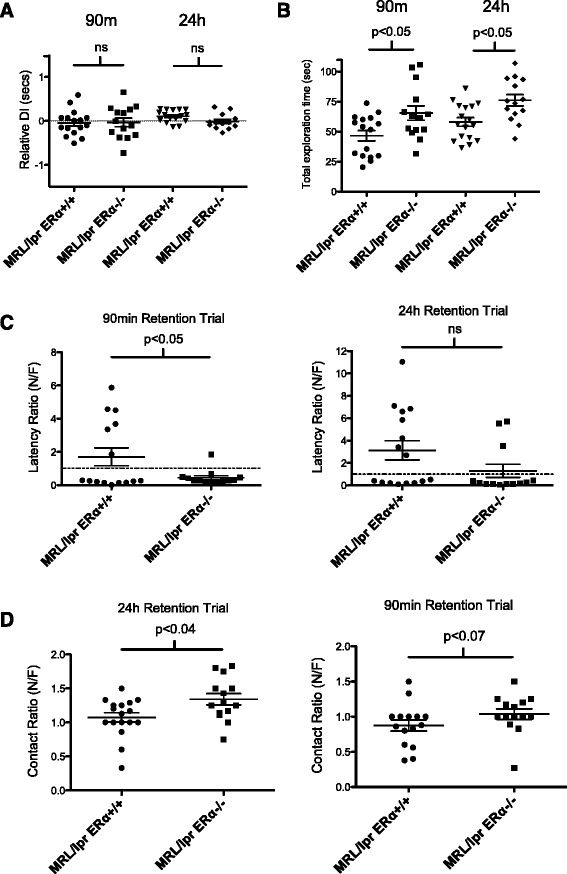
MRL/lpr ERαKO mice perform better than MRL/lpr WT in novel object recognition testing. (A) Relative discrimination (N-F/T) index. There was no significant difference between the discrimination ability of the MRL/lpr versus MRL/lpr ERαKO at 90 minutes or 24 hours. Neither group could effectively discriminate between the novel and familiar objects by this measure. A relative discrimination index of 0% (dotted line) indicates chance performance. (B) There was a significant difference in total exploration times between MRL/lpr and MRL/lpr ERαKO mice in both 90-minute and 24-hour trials. Post-hoc analysis revealed the total exploration time in both T1 and T2 of MRL/lpr ERαKO mice was higher than the total exploration time of MRL/lpr mice. (C) The latency ratio (time to first contact) was significantly decreased in the MRL/lpr ERαKO versus MRL/lpr mice at 90 minutes. There was also a trend towards improved performance in MRL/lpr ERαKO at 24 hours. (D) Contact ratio: MRL/lpr ERαKO mice made significantly more nose contacts with the novel object than the familiar object at 24 hours, and at 90 minutes there was a trend towards significance.
Figure 5
Hippocampal NeuN staining - MRL/lpr and MRL/lpr ERαKO mice. Selected images of NeuN immunostaining in the hippocampus of MRL/lpr and MRL/lpr ERαKO mice showing CA1 and dentate regions of interest (n = six to eight per group evaluated, three representative animals shown). There were no significant differences in hippocampal structure or volume noted. Scale bar in lower right corner represents 150 microns.
Figure 6
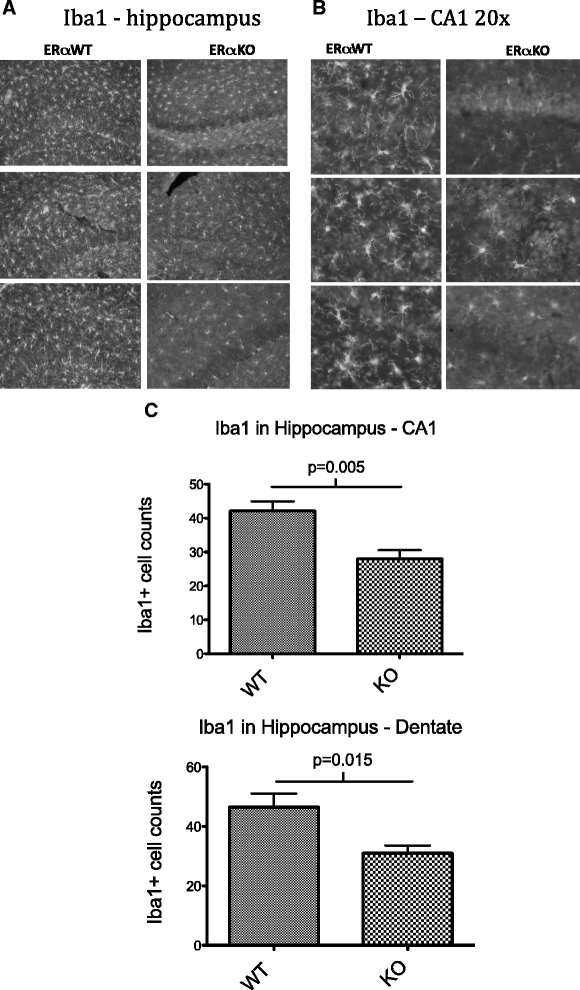
Hippocampal Iba1 staining in MRL/lpr and MRL/lpr ERαKO mice. (A) IHC images demonstrate diffuse glial activation and clustered microglia in the hippocampus of both MRL/lpr and MRL/lpr ERαKO mice. Scale bar in lower right corner represents 100 microns. (B) Higher magnification (20×) images demonstrating increased Iba1 immunopositive cells in MRL/lpr WT versus ERαKO mice. Scale bar in lower right corner represents 50 microns. (C) Counts of Iba1+ cells in CA1 and dentate regions of hippocampus showing a significant reduction in Iba1+ cells in MRL/lpr ERαKO mice (n = six to eight mice per group, average of three random fields each).
Similar articles
- Constitutive knockout of interleukin-6 ameliorates memory deficits and entorhinal astrocytosis in the MRL/lpr mouse model of neuropsychiatric lupus.
Reynolds J, Huang M, Li Y, Meineck M, Moeckel T, Weinmann-Menke J, Mohan C, Schwarting A, Putterman C. Reynolds J, et al. J Neuroinflammation. 2024 Apr 10;21(1):89. doi: 10.1186/s12974-024-03085-9. J Neuroinflammation. 2024. PMID: 38600510 Free PMC article. - B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus.
Wen J, Doerner J, Chalmers S, Stock A, Wang H, Gullinello M, Shlomchik MJ, Putterman C. Wen J, et al. J Neuroinflammation. 2016 Apr 7;13(1):73. doi: 10.1186/s12974-016-0537-3. J Neuroinflammation. 2016. PMID: 27055816 Free PMC article. - Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice.
Stock AD, Wen J, Doerner J, Herlitz LC, Gulinello M, Putterman C. Stock AD, et al. J Neuroinflammation. 2015 Nov 6;12:205. doi: 10.1186/s12974-015-0423-4. J Neuroinflammation. 2015. PMID: 26546449 Free PMC article. - Pyruvate kinase isoform M2 impairs cognition in systemic lupus erythematosus by promoting microglial synaptic pruning via the β-catenin signaling pathway.
Lu L, Wang H, Liu X, Tan L, Qiao X, Ni J, Sun Y, Liang J, Hou Y, Dou H. Lu L, et al. J Neuroinflammation. 2021 Oct 13;18(1):229. doi: 10.1186/s12974-021-02279-9. J Neuroinflammation. 2021. PMID: 34645459 Free PMC article. - Estrogen and progesterone receptors in murine models of systemic lupus erythematosus.
Greenstein B, Roa R, Dhaher Y, Nunn E, Greenstein A, Khamashta M, Hughes GR. Greenstein B, et al. Int Immunopharmacol. 2001 Jun;1(6):1025-35. doi: 10.1016/s1567-5769(01)00034-0. Int Immunopharmacol. 2001. PMID: 11407299 Review.
Cited by
- Estrogens, Neuroinflammation, and Neurodegeneration.
Villa A, Vegeto E, Poletti A, Maggi A. Villa A, et al. Endocr Rev. 2016 Aug;37(4):372-402. doi: 10.1210/er.2016-1007. Epub 2016 May 19. Endocr Rev. 2016. PMID: 27196727 Free PMC article. Review. - Updated advances of linking psychosocial factors and sex hormones with systemic lupus erythematosus susceptibility and development.
Pan Q, Chen X, Liao S, Chen X, Zhao C, Xu YZ, Liu HF. Pan Q, et al. PeerJ. 2019 Jun 25;7:e7179. doi: 10.7717/peerj.7179. eCollection 2019. PeerJ. 2019. PMID: 31275761 Free PMC article. - The Role of Steroid Hormones in the Modulation of Neuroinflammation by Dietary Interventions.
Vasconcelos AR, Cabral-Costa JV, Mazucanti CH, Scavone C, Kawamoto EM. Vasconcelos AR, et al. Front Endocrinol (Lausanne). 2016 Feb 4;7:9. doi: 10.3389/fendo.2016.00009. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 26869995 Free PMC article. Review. - Estrogen receptor alpha promotes lupus in (NZB×NZW)F1 mice in a B cell intrinsic manner.
Tabor DE, Gould KA. Tabor DE, et al. Clin Immunol. 2017 Jan;174:41-52. doi: 10.1016/j.clim.2016.10.011. Epub 2016 Oct 29. Clin Immunol. 2017. PMID: 27989899 Free PMC article. - Preconditioning with CpG-ODN1826 reduces ischemic brain injury in young male mice: a replication study.
Dave KR, Saul I, Raval AP, Perez-Pinzon MA. Dave KR, et al. Cond Med. 2019;2(4):178-184. Cond Med. 2019. PMID: 32510041 Free PMC article.
References
- The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes.Arthritis Rheum 1999, 42:599-608. - PubMed
- Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, Valdez CR, Escalante A, del Rincon I, Gronseth G, Rhine CB, Padilla P, McGlasson D. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58:1214–1220. doi: 10.1212/WNL.58.8.1214. - DOI - PubMed
- Sabbadini MG, Manfredi AA, Bozzolo E, Ferrario L, Rugarli C, Scorza R, Origgi L, Vanoli M, Gambini O, Vanzulli L, Croce D, Campana A, Messa C, Fazio F, Tincani A, Anzola G, Cattaneo R, Padovani A, Gasparotti R, Gerli R, Quartesan R, Piccirilli M, Farsi A, Emmi E, Passaleva A, Domeneghetti M, Piccini C, Massacesi L, Pupi A, De Cristoforis M, Danieli M, Candela P, Fraticelli M, Bartolini U, Salvolini G, Danieli G, Passaleva A. Central nervous system involvement in systemic lupus erythematosus patients without overt neuropsychiatric manifestations. Lupus. 1999;8:11–19. doi: 10.1191/096120399678847344. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR029880/RR/NCRR NIH HHS/United States
- KL2 RR029880/RR/NCRR NIH HHS/United States
- I01 BX000470/BX/BLRD VA/United States
- UL1 TR000062/TR/NCATS NIH HHS/United States
- KL2 TR000060/TR/NCATS NIH HHS/United States
- UL1 RR029882/RR/NCRR NIH HHS/United States
- P50 DA016511/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous