Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol - PubMed (original) (raw)
Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol
Chongben Zhang et al. J Biol Chem. 2015.
Abstract
Although an elevated triacylglycerol content in non-adipose tissues is often associated with insulin resistance, the mechanistic relationship remains unclear. The data support roles for intermediates in the glycerol-3-phosphate pathway of triacylglycerol synthesis: diacylglycerol (DAG), which may cause insulin resistance in liver by activating PKCϵ, and phosphatidic acid (PA), which inhibits insulin action in hepatocytes by disrupting the assembly of mTOR and rictor. To determine whether increases in DAG and PA impair insulin signaling when produced by pathways other than that of de novo synthesis, we examined primary mouse hepatocytes after enzymatically manipulating the cellular content of DAG or PA. Overexpressing phospholipase D1 or phospholipase D2 inhibited insulin signaling and was accompanied by an elevated cellular content of total PA, without a change in total DAG. Overexpression of diacylglycerol kinase-θ inhibited insulin signaling and was accompanied by an elevated cellular content of total PA and a decreased cellular content of total DAG. Overexpressing glycerol-3-phosphate acyltransferase-1 or -4 inhibited insulin signaling and increased the cellular content of both PA and DAG. Insulin signaling impairment caused by overexpression of phospholipase D1/D2 or diacylglycerol kinase-θ was always accompanied by disassociation of mTOR/rictor and reduction of mTORC2 kinase activity. However, although the protein ratio of membrane to cytosolic PKCϵ increased, PKC activity itself was unaltered. These data suggest that PA, but not DAG, is associated with impaired insulin action in mouse hepatocytes.
Keywords: Diacylglycerol Kinase; Glycerol-3-phosphate Acyltransferase; Glycerophospholipid; Insulin Resistance; Mammalian Target of Rapamycin (mTOR); Phospholipase D; Protein Kinase C (PKC).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
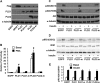
FIGURE 1.
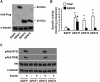
Overexpressing PLD1 and PLD2 impaired insulin signaling to Akt. Mouse primary hepatocytes were infected for 24 h with EGFP, Flag-GPAT1, PLD1, inactive PLD1 mutant (PLD1-m), PLD2, or inactive PLD2 mutant (PLD2-m) adenoviruses. Cells were lysed and subjected to Western blotting with appropriate antibodies (A), scraped from the dish, centrifuged to obtain total particulate preparations and assayed for PLD activity (B), or treated with or without insulin (100 n
m
) for 10 min, followed by cell lysate preparation for Western blotting (C and D). A, C, and D show representative Western blots from three independent experiments; B shows results from three independent experiments, each done in triplicate. E shows the quantitative analysis of data from D. * and #, p < 0.05 compared with EGFP basal and PMA-stimulated, respectively.
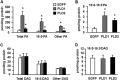
FIGURE 2.
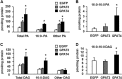
Overexpressing PLD1 and PLD2 increased the cellular content of total PA and di16:0 PA but did not change total or di16:0 DAG. Lipids were extracted from mouse primary hepatocytes overexpressing EGFP, PLD1, or PLD2. A, content of total PA, 16:0-containing PA, and other PA. B, content of di16:0 PA. C, content of total DAG, 16:0-containing DAG, and other DAG. D, content of di16:0 DAG. *, p < 0.05 compared with EGFP. The data are from three independent experiments, each done in triplicate.
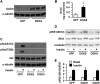
FIGURE 3.
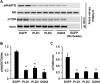
Overexpressing DGKθ impaired insulin signaling to Akt in mouse hepatocytes. Mouse primary hepatocytes were infected for 24 h with GFP or DGKθ lentiviruses. Cells were lysed and subjected to Western blotting with appropriate antibodies (A), lysed and assayed for DGK activity (B), or treated with or without insulin (100 n
m
) for 10 min, followed by cell lysate preparation for Western blotting (C and D). A, C, and D show representative Western blots from three independent experiments; B shows results from three independent experiments, each done in triplicate. E shows the quantitative analysis of data from D. *, p < 0.05 compared with GFP.
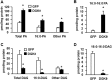
FIGURE 4.
Overexpressing DGKθ increased the cellular content of total PA and di16:0 PA and decreased total DAG and di16:0 DAG. Lipids were extracted from mouse primary hepatocytes overexpressing GFP or DGKθ, and lipid content was assayed. A, content of total PA, 16:0-containing PA, and other PA. B, content of di16:0 PA. C, content of total DAG, 16:0-containing DAG, and other DAG. D, content of di16:0 DAG. The data are from three independent experiments, each done in triplicate. *, p < 0.05 compared with GFP.
FIGURE 5.
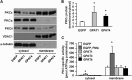
Overexpressed GPAT1 and GPAT4 in mouse hepatocytes, but not GPAT3, impaired insulin signaling to Akt. Mouse primary hepatocytes were infected for 24 h with EGFP, Flag-GPAT1, Flag-GPAT3, or Flag-GPAT4 adenoviruses. Cells were lysed and subjected to Western blotting with anti-Flag antibodies (A); scraped from the dish, centrifuged to obtain total particulate preparations, and assayed for total and NEM-resistant (NEM-R) GPAT activity (B); or treated with or without insulin (100 n
m
) for 10 min, followed by cell lysate preparation for Western blotting (C). A and C show representative Western blots from three independent experiments; B shows data from three independent experiments, each done in triplicate. * and #, p < 0.05 compared with EGFP total and NEM-resistant GPAT activity, respectively.
FIGURE 6.
Overexpressing GPAT4, but not GPAT3, increased the cellular content of total PA, DAG, and di16:0 PA. Lipids were extracted from mouse primary hepatocytes overexpressing EGFP, Flag-GPAT3, or Flag-GPAT4, and lipid content was assayed. A, content of total PA, 16:0-containing PA, and other PA. B, content of di16:0 PA. C, content of total DAG, 16:0-containing DAG, and other DAG. D, content of di16:0 DAG. The data are from three independent experiments, each done in triplicate. *, p < 0.05 compared with EGFP.
FIGURE 7.
Overexpressing PLD1, PLD2, or DGKθ diminished mTOR-rictor association and inhibited mTORC2 activity. Mouse primary hepatocytes overexpressing EGFP, PLD1, PLD2, or DGKθ were lysed and immunoprecipitated (IP) and then assayed for mTORC2 kinase activity followed by Western blotting. A, Western blots representative of three independent experiments. B and C, quantitative analysis of data from A. *, p < 0.05 compared with EGFP.
FIGURE 8.
Overexpressing GPAT1 or GPAT4 increased the protein ratio of membrane to cytosolic PKCϵ but did not alter PKC activity. Membrane and cytosolic preparations from mouse primary hepatocytes overexpressing EGFP, Flag-GPAT1, Flag-GPAT3, or Flag-GPAT4 were subjected to Western blotting (A) and protein quantification (B) or assayed for PKC activity (C). A and B show representative Western blots and protein quantifications from three independent experiments; C shows data from three independent experiments, each done in triplicate. *, p < 0.05 compared with EGFP.
Similar articles
- Glycerol-3-phosphate acyltransferase-4-deficient mice are protected from diet-induced insulin resistance by the enhanced association of mTOR and rictor.
Zhang C, Cooper DE, Grevengoed TJ, Li LO, Klett EL, Eaton JM, Harris TE, Coleman RA. Zhang C, et al. Am J Physiol Endocrinol Metab. 2014 Aug 1;307(3):E305-15. doi: 10.1152/ajpendo.00034.2014. Epub 2014 Jun 17. Am J Physiol Endocrinol Metab. 2014. PMID: 24939733 Free PMC article. - Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling.
Zhang C, Wendel AA, Keogh MR, Harris TE, Chen J, Coleman RA. Zhang C, et al. Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1667-72. doi: 10.1073/pnas.1110730109. Epub 2012 Jan 17. Proc Natl Acad Sci U S A. 2012. PMID: 22307628 Free PMC article. - Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR).
Foster DA, Salloum D, Menon D, Frias MA. Foster DA, et al. J Biol Chem. 2014 Aug 15;289(33):22583-22588. doi: 10.1074/jbc.R114.566091. Epub 2014 Jul 2. J Biol Chem. 2014. PMID: 24990952 Free PMC article. Review. - The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy.
You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, Hornberger TA. You JS, et al. J Biol Chem. 2014 Jan 17;289(3):1551-63. doi: 10.1074/jbc.M113.531392. Epub 2013 Dec 3. J Biol Chem. 2014. PMID: 24302719 Free PMC article. - Diacylglycerol, phosphatidic acid, and their metabolic enzymes in synaptic vesicle recycling.
Tu-Sekine B, Goldschmidt H, Raben DM. Tu-Sekine B, et al. Adv Biol Regul. 2015 Jan;57:147-52. doi: 10.1016/j.jbior.2014.09.010. Epub 2014 Sep 28. Adv Biol Regul. 2015. PMID: 25446883 Free PMC article. Review.
Cited by
- Lipidomic signatures in Colombian adults with metabolic syndrome.
Serna MF, Suarez-Ortegón MF, Jiménez-Charris E, Echeverri I, Cala MP, Mosquera M. Serna MF, et al. J Diabetes Metab Disord. 2024 May 4;23(1):1279-1292. doi: 10.1007/s40200-024-01423-5. eCollection 2024 Jun. J Diabetes Metab Disord. 2024. PMID: 38932852 Free PMC article. - GPAT1 Activity and Abundant Palmitic Acid Impair Insulin Suppression of Hepatic Glucose Production in Primary Mouse Hepatocytes.
Zhang C, Steadman M, Santos HP Jr, Shaikh SR, Xavier RM. Zhang C, et al. J Nutr. 2024 Apr;154(4):1109-1118. doi: 10.1016/j.tjnut.2024.02.004. Epub 2024 Feb 13. J Nutr. 2024. PMID: 38354952 - Non-Alcoholic Fatty Liver Disease or Type 2 Diabetes Mellitus-The Chicken or the Egg Dilemma.
Kosmalski M, Śliwińska A, Drzewoski J. Kosmalski M, et al. Biomedicines. 2023 Apr 4;11(4):1097. doi: 10.3390/biomedicines11041097. Biomedicines. 2023. PMID: 37189715 Free PMC article. Review. - New Era of Diacylglycerol Kinase, Phosphatidic Acid and Phosphatidic Acid-Binding Protein.
Sakane F, Hoshino F, Murakami C. Sakane F, et al. Int J Mol Sci. 2020 Sep 16;21(18):6794. doi: 10.3390/ijms21186794. Int J Mol Sci. 2020. PMID: 32947951 Free PMC article. Review. - Thioesterase superfamily member 2 promotes hepatic insulin resistance in the setting of glycerol-3-phosphate acyltransferase 1-induced steatosis.
Tillander V, Miniami A, Alves-Bezerra M, Coleman RA, Cohen DE. Tillander V, et al. J Biol Chem. 2019 Feb 8;294(6):2009-2020. doi: 10.1074/jbc.RA118.005184. Epub 2018 Dec 6. J Biol Chem. 2019. PMID: 30523156 Free PMC article.
References
- De Fea K., Roth R. A. (1997) Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36, 12939–12947 - PubMed
- Li Y., Soos T. J., Li X., Wu J., Degennaro M., Sun X., Littman D. R., Birnbaum M. J., Polakiewicz R. D. (2004) Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser1101. J. Biol. Chem. 279, 45304–45307 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL98050/HL/NHLBI NIH HHS/United States
- R01 DK101946/DK/NIDDK NIH HHS/United States
- R56 DK056598/DK/NIDDK NIH HHS/United States
- R01 DK056598/DK/NIDDK NIH HHS/United States
- P01 HL098050/HL/NHLBI NIH HHS/United States
- DK56598/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous