Repurposing of approved drugs from the human pharmacopoeia to target Wolbachia endosymbionts of onchocerciasis and lymphatic filariasis - PubMed (original) (raw)
Repurposing of approved drugs from the human pharmacopoeia to target Wolbachia endosymbionts of onchocerciasis and lymphatic filariasis
Kelly L Johnston et al. Int J Parasitol Drugs Drug Resist. 2014.
Abstract
Lymphatic filariasis and onchocerciasis are debilitating diseases caused by parasitic filarial nematodes infecting around 150 million people throughout the tropics with more than 1.5 billion at risk. As with other neglected tropical diseases, classical drug-discovery and development is lacking and a 50 year programme of macrofilaricidal discovery failed to deliver a drug which can be used as a public health tool. Recently, antibiotic targeting of filarial Wolbachia, an essential bacterial symbiont, has provided a novel drug treatment for filariasis with macrofilaricidal activity, although the current gold-standard, doxycycline, is unsuitable for use in mass drug administration (MDA). The anti-Wolbachia (A·WOL) Consortium aims to identify novel anti-Wolbachia drugs, compounds or combinations that are suitable for use in MDA. Development of a Wolbachia cell-based assay has enabled the screening of the approved human drug-pharmacopoeia (∼2600 drugs) for a potential repurposing. This screening strategy has revealed that approved drugs from various classes show significant bacterial load reduction equal to or superior to the gold-standard doxycycline, with 69 orally available hits from different drug categories being identified. Based on our defined hit criteria, 15 compounds were then selectively screened in a Litomosoides sigmodontis mouse model, 4 of which were active. These came from the tetracycline, fluoroquinolone and rifamycin classes. This strategy of repurposing approved drugs is a promising development in the goal of finding a novel treatment against filariasis and could also be a strategy applicable for other neglected tropical diseases.
Keywords: Anti-Wolbachia Consortium (A·WOL); Drug discovery; Filariasis; Library screening; Pharmacopoeia; Wolbachia.
Figures
Graphical abstract
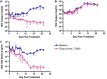
Fig. 1
Dynamics of cell and Wolbachia response to doxycycline over 21 days. Wolbachia growth was assessed by qPCR targeting the 16S rRNA gene (A). C6/36 cell growth was assessed by qPCR targeting the 18S rRNA gene of Aedes albopictus (B). Data was normalised using the ratio of 16S copies to 18S copies (C).
Similar articles
- Development and validation of a high-throughput anti-Wolbachia whole-cell screen: a route to macrofilaricidal drugs against onchocerciasis and lymphatic filariasis.
Clare RH, Cook DA, Johnston KL, Ford L, Ward SA, Taylor MJ. Clare RH, et al. J Biomol Screen. 2015 Jan;20(1):64-9. doi: 10.1177/1087057114551518. Epub 2014 Oct 2. J Biomol Screen. 2015. PMID: 25278497 - Combinations of registered drugs reduce treatment times required to deplete Wolbachia in the Litomosoides sigmodontis mouse model.
Specht S, Pfarr KM, Arriens S, Hübner MP, Klarmann-Schulz U, Koschel M, Sternberg S, Martin C, Ford L, Taylor MJ, Hoerauf A. Specht S, et al. PLoS Negl Trop Dis. 2018 Jan 4;12(1):e0006116. doi: 10.1371/journal.pntd.0006116. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29300732 Free PMC article. - Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis.
Taylor MJ, von Geldern TW, Ford L, Hübner MP, Marsh K, Johnston KL, Sjoberg HT, Specht S, Pionnier N, Tyrer HE, Clare RH, Cook DAN, Murphy E, Steven A, Archer J, Bloemker D, Lenz F, Koschel M, Ehrens A, Metuge HM, Chunda VC, Ndongmo Chounna PW, Njouendou AJ, Fombad FF, Carr R, Morton HE, Aljayyoussi G, Hoerauf A, Wanji S, Kempf DJ, Turner JD, Ward SA. Taylor MJ, et al. Sci Transl Med. 2019 Mar 13;11(483):eaau2086. doi: 10.1126/scitranslmed.aau2086. Sci Transl Med. 2019. PMID: 30867321 - Anti-Wolbachia drugs for filariasis.
Johnston KL, Hong WD, Turner JD, O'Neill PM, Ward SA, Taylor MJ. Johnston KL, et al. Trends Parasitol. 2021 Dec;37(12):1068-1081. doi: 10.1016/j.pt.2021.06.004. Epub 2021 Jul 3. Trends Parasitol. 2021. PMID: 34229954 Review. - Anti-Wolbachia therapy for onchocerciasis & lymphatic filariasis: Current perspectives.
Wan Sulaiman WA, Kamtchum-Tatuene J, Mohamed MH, Ramachandran V, Ching SM, Sazlly Lim SM, Hashim HZ, Inche Mat LN, Hoo FK, Basri H. Wan Sulaiman WA, et al. Indian J Med Res. 2019 Jun;149(6):706-714. doi: 10.4103/ijmr.IJMR_454_17. Indian J Med Res. 2019. PMID: 31496523 Free PMC article. Review.
Cited by
- Novel Findings of Anti-Filarial Drug Target and Structure-Based Virtual Screening for Drug Discovery.
Choi TW, Cho JH, Ahnn J, Song HO. Choi TW, et al. Int J Mol Sci. 2018 Nov 13;19(11):3579. doi: 10.3390/ijms19113579. Int J Mol Sci. 2018. PMID: 30428563 Free PMC article. - Wolbachia endosymbionts induce neutrophil extracellular trap formation in human onchocerciasis.
Tamarozzi F, Turner JD, Pionnier N, Midgley A, Guimaraes AF, Johnston KL, Edwards SW, Taylor MJ. Tamarozzi F, et al. Sci Rep. 2016 Oct 18;6:35559. doi: 10.1038/srep35559. Sci Rep. 2016. PMID: 27752109 Free PMC article. - Repurposing strategies for tropical disease drug discovery.
Klug DM, Gelb MH, Pollastri MP. Klug DM, et al. Bioorg Med Chem Lett. 2016 Jun 1;26(11):2569-76. doi: 10.1016/j.bmcl.2016.03.103. Epub 2016 Mar 30. Bioorg Med Chem Lett. 2016. PMID: 27080183 Free PMC article. Review. - The role of 'omics' in the quest to eliminate human filariasis.
Lustigman S, Grote A, Ghedin E. Lustigman S, et al. PLoS Negl Trop Dis. 2017 Apr 20;11(4):e0005464. doi: 10.1371/journal.pntd.0005464. eCollection 2017 Apr. PLoS Negl Trop Dis. 2017. PMID: 28426656 Free PMC article. Review. No abstract available. - Industrial scale high-throughput screening delivers multiple fast acting macrofilaricides.
Clare RH, Bardelle C, Harper P, Hong WD, Börjesson U, Johnston KL, Collier M, Myhill L, Cassidy A, Plant D, Plant H, Clark R, Cook DAN, Steven A, Archer J, McGillan P, Charoensutthivarakul S, Bibby J, Sharma R, Nixon GL, Slatko BE, Cantin L, Wu B, Turner J, Ford L, Rich K, Wigglesworth M, Berry NG, O'Neill PM, Taylor MJ, Ward SA. Clare RH, et al. Nat Commun. 2019 Jan 2;10(1):11. doi: 10.1038/s41467-018-07826-2. Nat Commun. 2019. PMID: 30602718 Free PMC article.
References
- Arumugam S., Pfarr K.M., Hoerauf A. Infection of the intermediate mite host with Wolbachia-depleted Litomosoides sigmodontis microfilariae: impaired L1 to L3 development and subsequent sex-ratio distortion in adult worms. Int. J. Parasitol. 2008;38:981–987. - PubMed
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. - PubMed
- Bockarie M.J., Deb R.M. Elimination of lymphatic filariasis: do we have the drugs to complete the job? Curr. Opin. Infect. Dis. 2010;23:617–620. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials