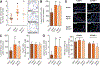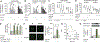Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment - PubMed (original) (raw)
. 2015 Feb 27;116(5):827-35.
doi: 10.1161/CIRCRESAHA.116.305825. Epub 2014 Dec 17.
Renske de Jong 1, Jan Rossaint 1, Joana R Viola 1, Giovanna Leoni 1, Ji Ming Wang 1, Jochen Grommes 1, Rabea Hinkel 1, Christian Kupatt 1, Christian Weber 1, Yvonne Döring 1, Alexander Zarbock 1, Oliver Soehnlein 2
Affiliations
- PMID: 25520364
- PMCID: PMC7751381
- DOI: 10.1161/CIRCRESAHA.116.305825
Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment
Maik Drechsler et al. Circ Res. 2015.
Abstract
Rationale: Chemokine-controlled arterial leukocyte recruitment is a crucial process in atherosclerosis. Formyl peptide receptor 2 (FPR2) is a chemoattractant receptor that recognizes proinflammatory and proresolving ligands. The contribution of FPR2 and its proresolving ligand annexin A1 to atherosclerotic lesion formation is largely undefined.
Objective: Because of the ambivalence of FPR2 ligands, we here investigate the role of FPR2 and its resolving ligand annexin A1 in atherogenesis.
Methods and results: Deletion of FPR2 or its ligand annexin A1 enhances atherosclerotic lesion formation, arterial myeloid cell adhesion, and recruitment. Mechanistically, we identify annexin A1 as an endogenous inhibitor of integrin activation evoked by the chemokines CCL5, CCL2, and CXCL1. Specifically, the annexin A1 fragment Ac2-26 counteracts conformational activation and clustering of integrins on myeloid cells evoked by CCL5, CCL2, and CXCL1 through inhibiting activation of the small GTPase Rap1. In vivo administration of Ac2-26 largely diminishes arterial recruitment of myeloid cells in a FPR2-dependent fashion. This effect is also observed in the presence of selective antagonists to CCR5, CCR2, or CXCR2, whereas Ac2-26 was without effect when all 3 chemokine receptors were antagonized simultaneously. Finally, repeated treatment with Ac2-26 reduces atherosclerotic lesion sizes and lesional macrophage accumulation.
Conclusions: Instructing the annexin A1-FPR2 axis harbors a novel approach to target arterial leukocyte recruitment. With the ability of Ac2-26 to counteract integrin activation exerted by various chemokines, delivery of Ac2-26 may be superior in inhibition of arterial leukocyte recruitment when compared with blocking individual chemokine receptors.
Keywords: annexin A1; atherosclerosis; chemokine; leukocytes.
© 2014 American Heart Association, Inc.
Figures
Figure 1.. Formyl peptide receptor 2 and its ligand annexin A1 protect from atherogenesis.
_Apoe_−/−, _Apoe_−/−_Fpr2_−/−, and _Apoe_−/−_Anxa1_−/− mice were fed a high-fat diet for 4 weeks. A, Quantification of atherosclerotic lesion sizes in Oil-Red-O–stained aortic root sections. Representative images are shown aside. B, Enumeration of lesional DAPI (4’,6-diamidino-2-phenylindole)-positive cells. C, Quantification of Mac2+ cells indicating macrophage accumulation in absolute numbers (left) and relative to lesional cell count (right). D, Quantification of lesional Ly6G+ neutrophils. E, Assessment of luminal expression of endothelial adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Displayed are representative images (top) and quantification of luminal coverage (bottom). Scale bar, 100 μm. *P<0.05 compared to _Apoe_−/− mice. All data are presented as mean±SD. Experiments were performed 3× independently with a total of 15 mice. Data were analyzed with 1-way ANOVA with Dunnett post test.
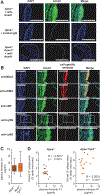
Figure 2.. Plasma annexin A1 (AnxA1) negatively correlates with atherosclerotic lesion sizes.
A, _Apoe_−/− and _Apoe_−/−_Anxa1_−/− mice were fed a high-fat diet for 4 weeks, and aortic root sections were stained with an antibody to AnxA1 or an isotype control antibody. B, _Apoe_−/− mice were fed a high-fat diet (HFD) for 4 weeks and AnxA1 was costained with markers for macrophages (anti-Mac2), smooth muscle cells (anti-αSMA), endothelial cells (anti-von Willebrand Factor [vWF]), or neutrophils (anti-Ly6G) in aortic root sections. Scale bar, 100 μm. C and D, _Apoe_−/− and _Apoe_−/−_Fpr2_−/− mice were fed a HFD for 4 weeks and AnxA1 was quantified in the plasma (B). Correlation between aortic root lesion sizes and plasma AnxA1 levels in _Apoe_−/− and _Apoe_−/−_Fpr2_−/− mice (Pearson correlation; D). DAPI indicates 4’,6-diamidino-2-phenylindole.
Figure 3.. Annexin A1-formyl peptide receptor 2 axis prevents arterial myeloid cell recruitment.
_Apoe_−/−, _Apoe_−/−_Fpr2_−/−, and _Apoe_−/−_Anxa1_−/− mice were fed a high-fat diet for 4 weeks, and intravital microscopy of the carotid artery was performed to assess luminal leukocyte endothelial interactions. Myeloid cell subsets were identified by intravenous injection of antibodies to Ly6G, Ly6C, and CD11b 10 minutes before recording. Displayed are quantification of tethering (A), rolling speed (B), and rolling flux (C) of CD11b+ cells. Number of adherent cells (E) is displayed for CD11b+ cells (left), Ly6G+ cells (middle), and Ly6C+ cells (right). Adhesion of CD11b+ myeloid cells to carotid arteries of _Apoe_−/−, _Apoe_−/−_Fpr2_−/−, and _Apoe_−/−_Anxa1_−/− mice is depicted in D. Scale bar, 100 μm. *P<0.05 compared with _Apoe_−/− mice. Experiments were performed 3× independently with a total of ≥15 mice. Data were analyzed using 1-way ANOVA with Dunnett post test.
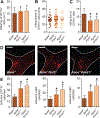
Figure 4.. Annexin A1 counteracts chemokine-induced integrin activation.
A and B, Annexin A1 inhibits chemokine-evoked integrin activation on myeloid cells. Neutrophils (A) and classical monocytes (B) from C57Bl/6 mice were treated with CCL2 or CCL5, and binding of soluble vascular cell adhesion molecule-1 (VCAM-1)-Fc or ICAM-1-Fc was assessed by flow cytometry. Effect of Ac2-26 was determined by pretreatment for 30 minutes before chemokine stimulation. C and D, Dose-dependent response of Ac2-26 on CCL5-evoked binding of VCAM-1-Fc or intercellular adhesion molecule-1 (ICAM-1)-Fc on neutrophils (C) and monocytes (D) harvested from C57Bl/6 (wild-type [WT]) or _Fpr2_−/− mice. E, β2 integrin activation assessed by mAb24 binding and flow cytometry in human neutrophils incubated with CCL5 and preincubated with Ac2-26. Where indicated, neutrophils were pretreated with WT or constitutively active (CA) Rap1-Tat peptides. F, Redistribution of surface LFA1 on mouse neutrophils stimulated for 90 s with CCL5 and pretreated for 30 minutes with Ac2-26. G, Mean fluorescence intensity (MFI) reflecting intracellular calcium in mouse neutrophils before and after addition of CCL5 with or without Ac2-26, which was added 30 minutes before CCL5 stimulation. Data are representative recordings from 5 independent runs. H, GTP-bound Rap1 protein in mouse neutrophils 30 s after stimulation with CCL5 and prestimulation for 30 minutes with Ac2-26. Representative blots from 3 experiments are shown. All data are presented as mean±SD. Experiments were performed ≥6× independently for A–E. *P<0.05 in C and D *Differences compared with CCL5 stimulation. Data were analyzed using Kruskal–Wallis test with Dunn post test. CAM indicates cell adhesion molecule.
Figure 5.. In vivo delivery of the annexin A1 fragment Ac2-26 reduces atherosclerosis.
A–C, _Apoe_−/−, _Apoe_−/−_Fpr2_−/−, and _Apoe_−/−_Anxa1_−/− mice were fed a high-fat diet (HFD) for 4 weeks. Intravital microscopy of the carotid artery was used for assessment of luminal leukocyte endothelial interactions. Myeloid cells were identified by intravenous injection of an antibody to CD11b 10 minutes before recording. Myeloid cell adhesion (B) and rolling flux (C) in _Apoe_−/− (left), _Apoe_−/−_Fpr2_−/− (middle), and _Apoe_−/−_Anxa1_−/− (right) mice were assessed before and 30 minutes after injection of native (panels to the left) or boiled Ac2-26 (far right panel; 50 μg, IV). Representative images are shown in A. Scale bar, 100 μm. Each dot represents 1 mouse. *P<0.05 compared with read-out before Ac2-26 administration. Data were analyzed with paired t test. D, _Apoe_−/− mice were fed a HFD for 4 weeks and mice received a single dose of antagonist to CCR2 (RS504393, 5 mg/kg), CCR5 (DAPTA, 1 mg/kg), or CXCR2 (SB225002, 5 mg/kg), or a combination of all, or vehicle control. Thirty minutes later arterial adhesion of CD11b+ cells was studied (before). Immediately after recording, mice received Ac2-26 (50 μg IV) and adhesion was studied 30 minutes later (after). Each dot represents 1 mouse. *P<0.05 compared with read-out before Ac2-26 administration. Data were analyzed with Wilcoxon matched-pairs signed rank test. E–G, Ac2-26 reduces atherogenesis. _Apoe_−/− mice (n=6 per group) were repeatedly injected with Ac2-26 (3× per week; 50 μg IP per injection) or vehicle control during 4 weeks of HFD. E, Quantification of atherosclerotic lesion sizes in Oil-Red-O–stained aortic root sections. Representative images are shown aside. F, Quantification of Mac2+ macrophages. G, Quantification of lesional Ly6G+ neutrophils. *P<0.05 compared with vehicle treatment. Data in F and G are presented as mean±SD. Data in E–G were analyzed with Mann-Whitney test.
Comment in
- wRAPping up early monocyte and neutrophil recruitment in atherogenesis via Annexin A1/FPR2 signaling.
Butcher MJ, Galkina EV. Butcher MJ, et al. Circ Res. 2015 Feb 27;116(5):774-7. doi: 10.1161/CIRCRESAHA.115.305920. Circ Res. 2015. PMID: 25722438 Free PMC article. No abstract available.
Similar articles
- Deficiency of the sialyltransferase St3Gal4 reduces Ccl5-mediated myeloid cell recruitment and arrest: short communication.
Döring Y, Noels H, Mandl M, Kramp B, Neideck C, Lievens D, Drechsler M, Megens RT, Tilstam PV, Langer M, Hartwig H, Theelen W, Marth JD, Sperandio M, Soehnlein O, Weber C. Döring Y, et al. Circ Res. 2014 Mar 14;114(6):976-81. doi: 10.1161/CIRCRESAHA.114.302426. Epub 2014 Jan 14. Circ Res. 2014. PMID: 24425712 Free PMC article. - Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation.
Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D'Acquisto F, Buckingham JC, Perretti M, Flower RJ. Dufton N, et al. J Immunol. 2010 Mar 1;184(5):2611-2619. doi: 10.4049/jimmunol.0903526. Epub 2010 Jan 27. J Immunol. 2010. PMID: 20107188 Free PMC article. - Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes.
Soehnlein O, Drechsler M, Döring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gómez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Soehnlein O, et al. EMBO Mol Med. 2013 Mar;5(3):471-81. doi: 10.1002/emmm.201201717. Epub 2013 Feb 18. EMBO Mol Med. 2013. PMID: 23417922 Free PMC article. - Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks.
Semple BD, Kossmann T, Morganti-Kossmann MC. Semple BD, et al. J Cereb Blood Flow Metab. 2010 Mar;30(3):459-73. doi: 10.1038/jcbfm.2009.240. Epub 2009 Nov 11. J Cereb Blood Flow Metab. 2010. PMID: 19904283 Free PMC article. Review. - Annexin A1: potential for glucocorticoid sparing in RA.
Yang YH, Morand E, Leech M. Yang YH, et al. Nat Rev Rheumatol. 2013 Oct;9(10):595-603. doi: 10.1038/nrrheum.2013.126. Epub 2013 Aug 20. Nat Rev Rheumatol. 2013. PMID: 23958797 Review.
Cited by
- Stimulation of the Pro-Resolving Receptor Fpr2 Reverses Inflammatory Microglial Activity by Suppressing NFκB Activity.
Wickstead ES, Elliott BT, Pokorny S, Biggs C, Getting SJ, McArthur S. Wickstead ES, et al. Int J Mol Sci. 2023 Nov 6;24(21):15996. doi: 10.3390/ijms242115996. Int J Mol Sci. 2023. PMID: 37958978 Free PMC article. - Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR-/- Mice on Western Type Diet.
Kusters DH, Chatrou ML, Willems BA, De Saint-Hubert M, Bauwens M, van der Vorst E, Bena S, Biessen EA, Perretti M, Schurgers LJ, Reutelingsperger CP. Kusters DH, et al. PLoS One. 2015 Jun 19;10(6):e0130484. doi: 10.1371/journal.pone.0130484. eCollection 2015. PLoS One. 2015. PMID: 26090792 Free PMC article. - Myeloid-Specific Deletion of Peptidylarginine Deiminase 4 Mitigates Atherosclerosis.
Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, Yang ZH, Hemmers S, Remaley AT, Mowen KA, Kaplan MJ. Liu Y, et al. Front Immunol. 2018 Jul 26;9:1680. doi: 10.3389/fimmu.2018.01680. eCollection 2018. Front Immunol. 2018. PMID: 30140264 Free PMC article. - Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease.
Pirault J, Bäck M. Pirault J, et al. Front Pharmacol. 2018 Nov 14;9:1273. doi: 10.3389/fphar.2018.01273. eCollection 2018. Front Pharmacol. 2018. PMID: 30487747 Free PMC article. Review. - Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance.
Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Sugimoto MA, et al. J Immunol Res. 2016;2016:8239258. doi: 10.1155/2016/8239258. Epub 2016 Jan 13. J Immunol Res. 2016. PMID: 26885535 Free PMC article. Review.
References
- Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases