Possible adverse effects of SGLT2 inhibitors on bone - PubMed (original) (raw)
Possible adverse effects of SGLT2 inhibitors on bone
Simeon I Taylor et al. Lancet Diabetes Endocrinol. 2015 Jan.
No abstract available
Conflict of interest statement
Conflicts of interest
SIT was previously employed by and currently owns restricted stock in the Bristol-Myers Squibb Company. In addition, he is a paid consultant and adviser for the following companies: Aegerion Pharmaceuticals, Inc.; Ambrx, Inc.; Calibrium, LLC; Genkyotex, S.A.; Isis Pharmaceuticals, Inc.; Nimbus Discovery, LLC; Yabao Pharmaceutical Co., Ltd.; and Cadila Healthcare Limited. Neither JEB nor KIR have any conflicts of interest to report.
Figures
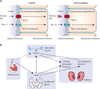
Figure 1. Proposed mechanisms whereby SGTL2 inhibitors exert adverse effects on bone
Panel A: Published data demonstrate that SGLT2 inhibitors increase serum phosphate levels,, probably by promoting renal tubular phosphate reabsorption. Because of the action of SGLT2 inhibitors to decrease Na+ transport, this increases the electrochemical gradient for Na+, thereby driving increased co-transport of phosphate and Na+. In human studies, phlorizin (a non-selective SGLT1/ SGLT2 inhibitor) decreased phosphate clearance by an average of ~80% during the first hour after phlorizin administration. Furthermore, phlorizin promotes phosphate reabsorption in perfused proximal convoluted tubules. This is likely the mechanism of the observed SGLT2 inhibitor-induced increase in serum phosphate. Panel B: Increased levels of serum phosphate are predicted to increase secretion of PTH by the parathyroid gland. Either directly or indirectly (e.g., mediated by effects of PTH), the increased serum phosphate has the potential to increase FGF23 secretion by osteocytes in bone.– The effect of phosphate to promote PTH secretion is believed to be mediated, at least in part, by the effect of increased phosphate levels to decrease levels of free ionized Ca++. Both PTH and FGF23 promote phosphaturia by decreasing renal tubular reabsorption of phosphate. In contrast, the two hormones exert opposite effects upon 1α-hydroxylation of 25-hydroxyvitamin D – with PTH increasing and FGF23 decreasing 1,25-dihydroxyvitamin D formation.
Similar articles
- Pharmacological aspects of the safety of gliflozins.
Faillie JL. Faillie JL. Pharmacol Res. 2017 Apr;118:71-81. doi: 10.1016/j.phrs.2016.07.001. Epub 2016 Jul 5. Pharmacol Res. 2017. PMID: 27389050 Review. - Bone Fractures with Sodium-Glucose Co-transporter-2 Inhibitors: How Real is the Risk?
Mannucci E, Monami M. Mannucci E, et al. Drug Saf. 2017 Feb;40(2):115-119. doi: 10.1007/s40264-016-0470-5. Drug Saf. 2017. PMID: 27826881 Review. - Osmotic diuresis with SGLT2 inhibition: analysis of events related to volume reduction in dapagliflozin clinical trials.
Johnsson K, Johnsson E, Mansfield TA, Yavin Y, Ptaszynska A, Parikh SJ. Johnsson K, et al. Postgrad Med. 2016 May;128(4):346-55. doi: 10.1080/00325481.2016.1153941. Epub 2016 Mar 2. Postgrad Med. 2016. PMID: 26878357 - In brief: ketoacidosis with SGLT2 inhibitors.
[No authors listed] [No authors listed] Med Lett Drugs Ther. 2015 Jun 22;57(1471):94. Med Lett Drugs Ther. 2015. PMID: 26079766 No abstract available. - [Acidosis without marked hyperglycemia : Euglycemic diabetic ketoacidosis associated with SGLT2-Inhibitors].
Valek R, Von der Mark J. Valek R, et al. Med Klin Intensivmed Notfmed. 2017 Mar;112(2):145-148. doi: 10.1007/s00063-016-0153-0. Epub 2016 May 24. Med Klin Intensivmed Notfmed. 2017. PMID: 27221094 German.
Cited by
- The effect of empagliflozin (sodium-glucose cotransporter-2 inhibitor) on osteoporosis and glycemic parameters in patients with type 2 diabetes: a quasi-experimental study.
Tan J, Guo A, Zhang K, Jiang Y, Liu H. Tan J, et al. BMC Musculoskelet Disord. 2024 Oct 7;25(1):793. doi: 10.1186/s12891-024-07900-5. BMC Musculoskelet Disord. 2024. PMID: 39375646 Free PMC article. Clinical Trial. - Modern Challenges in Type 2 Diabetes: Balancing New Medications with Multifactorial Care.
Caturano A, Galiero R, Rocco M, Tagliaferri G, Piacevole A, Nilo D, Di Lorenzo G, Sardu C, Vetrano E, Monda M, Marfella R, Rinaldi L, Sasso FC. Caturano A, et al. Biomedicines. 2024 Sep 7;12(9):2039. doi: 10.3390/biomedicines12092039. Biomedicines. 2024. PMID: 39335551 Free PMC article. Review. - Antidiabetic Agents and Bone Quality: A Focus on Glycation End Products and Incretin Pathway Modulations.
Zaki MK, Abed MN, Alassaf FA. Zaki MK, et al. J Bone Metab. 2024 Aug;31(3):169-181. doi: 10.11005/jbm.2024.31.3.169. Epub 2024 Aug 31. J Bone Metab. 2024. PMID: 39307518 Free PMC article. - A Randomized Controlled Trial on the Effect of Luseogliflozin on Bone Microarchitecture Evaluated Using HR-pQCT in Elderly Type 2 Diabetes.
Shigeno R, Horie I, Haraguchi A, Niimi R, Chiba K, Tashiro S, Kawazoe Y, Sato S, Osaki M, Kawakami A, Abiru N. Shigeno R, et al. Diabetes Ther. 2024 Oct;15(10):2233-2248. doi: 10.1007/s13300-024-01634-2. Epub 2024 Aug 17. Diabetes Ther. 2024. PMID: 39153152 Free PMC article. - Potential Metabolic Pathways Involved in Osteoporosis and Evaluation of Fracture Risk in Individuals with Diabetes.
Liu T, Wang Y, Qian B, Li P. Liu T, et al. Biomed Res Int. 2024 May 23;2024:6640796. doi: 10.1155/2024/6640796. eCollection 2024. Biomed Res Int. 2024. PMID: 38884020 Free PMC article. Review.
References
- Tahrani A, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140–151. - PubMed
- Kwon H. Canagliflozin: clinical efficacy and safety. Endocrinology and Metabolic Drugs Advisory Committee Meeting. 2013 www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug....
- Bristol-Myers Squibb Company, Astra Zeneca AB. Background Document: Dapagliflozin. 2011 www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug....
- Skeith MD, Healey LA, Cutler RE. Effect of phloridzin on uric acid excretion in man. Amer J Physiol. 1970;219:1080–1082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical