Sex differences in normal age trajectories of functional brain networks - PubMed (original) (raw)
. 2015 Apr;36(4):1524-35.
doi: 10.1002/hbm.22720. Epub 2014 Dec 18.
Affiliations
- PMID: 25523617
- PMCID: PMC5522589
- DOI: 10.1002/hbm.22720
Sex differences in normal age trajectories of functional brain networks
Dustin Scheinost et al. Hum Brain Mapp. 2015 Apr.
Abstract
Resting-state functional magnetic resonance image (rs-fMRI) is increasingly used to study functional brain networks. Nevertheless, variability in these networks due to factors such as sex and aging is not fully understood. This study explored sex differences in normal age trajectories of resting-state networks (RSNs) using a novel voxel-wise measure of functional connectivity, the intrinsic connectivity distribution (ICD). Males and females showed differential patterns of changing connectivity in large-scale RSNs during normal aging from early adulthood to late middle-age. In some networks, such as the default-mode network, males and females both showed decreases in connectivity with age, albeit at different rates. In other networks, such as the fronto-parietal network, males and females showed divergent connectivity trajectories with age. Main effects of sex and age were found in many of the same regions showing sex-related differences in aging. Finally, these sex differences in aging trajectories were robust to choice of preprocessing strategy, such as global signal regression. Our findings resolve some discrepancies in the literature, especially with respect to the trajectory of connectivity in the default mode, which can be explained by our observed interactions between sex and aging. Overall, results indicate that RSNs show different aging trajectories for males and females. Characterizing effects of sex and age on RSNs are critical first steps in understanding the functional organization of the human brain.
Keywords: aging; brain networks; functional connectivity; resting state; sex differences.
© 2014 Wiley Periodicals, Inc.
Figures
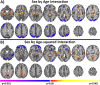
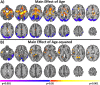
Figure 1
Sex by aging interaction for connectivity. Sex by age interactions are shown in (A) and sex by age‐squared interactions are shown in (B). Widespread significant (P < 0.05 corrected) differences in aging trajectories between males and females were observed. Warm colors represent areas where the slope associated with age or age‐squared is greater for females compared to males. Cool colors represent areas where the slope associated with age or age‐squared is greater for males compared to females. [Color figure can be viewed in the online issue, which is available at
.]
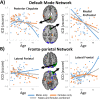
Figure 2
Scatterplots for sex by aging interaction in (A) DMN and (B) FPN. (A) For both DMN nodes, males displayed a greater change in connectivity per yearly increase in age compared to females. (B) For both FPN nodes, males and females showed divergent directions of aging trajectories with males showing increased connectivity with age and females showing decreased connectivity with age. [Color figure can be viewed in the online issue, which is available at
.]
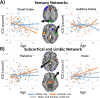
Figure 3
Scatterplots for sex by aging interaction in (A) sensory and (B) subcortical and limbic networks. (A) Sensory networks displayed sex by aging interaction in the visual and auditory networks. (B) Females showed increased connectivity with age in many subcortical and limbic regions, whereas males showed little change in connectivity with age. [Color figure can be viewed in the online issue, which is available at
.]
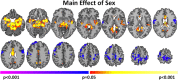
Figure 4
Sex Differences in connectivity. Widespread significant (P < 0.05 corrected) differences between males and females were observed. Warm colors represent areas with greater connectivity for females compared with males. Cool colors represent areas with greater connectivity for males compared with females. [Color figure can be viewed in the online issue, which is available at
.]
Figure 5
Linear and non‐linear effects of aging on connectivity. Widespread significant (P < 0.05 corrected) correlations with age and age‐squared were observed. (A) Correlations with age. (B) Correlations with age‐squared. These maps are collapsed across males and females. Warm colors represent areas with a positive correlation with age or age‐squared. Cool colors represent areas with negative correlations with age or age‐squared. [Color figure can be viewed in the online issue, which is available at
.]
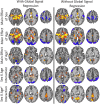
Figure 6
Robustness of results to preprocessing. The observe sex differences in aging trajectories were robust to choice of preprocessing strategy. The results with (right column) and without GSR (left column) are largely the same. [Color figure can be viewed in the online issue, which is available at
.]
Similar articles
- Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults.
Mowinckel AM, Espeseth T, Westlye LT. Mowinckel AM, et al. Neuroimage. 2012 Nov 15;63(3):1364-73. doi: 10.1016/j.neuroimage.2012.08.004. Epub 2012 Aug 10. Neuroimage. 2012. PMID: 22992492 - A Brain-Wide Study of Age-Related Changes in Functional Connectivity.
Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. Geerligs L, et al. Cereb Cortex. 2015 Jul;25(7):1987-99. doi: 10.1093/cercor/bhu012. Epub 2014 Feb 13. Cereb Cortex. 2015. PMID: 24532319 - Manipulating brain connectivity with δ⁹-tetrahydrocannabinol: a pharmacological resting state FMRI study.
Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, Te Beek ET, Rombouts SA, van Gerven JM. Klumpers LE, et al. Neuroimage. 2012 Nov 15;63(3):1701-11. doi: 10.1016/j.neuroimage.2012.07.051. Epub 2012 Aug 1. Neuroimage. 2012. PMID: 22885247 Clinical Trial. - The Default Mode Network in Healthy Individuals: A Systematic Review and Meta-Analysis.
Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R. Mak LE, et al. Brain Connect. 2017 Feb;7(1):25-33. doi: 10.1089/brain.2016.0438. Epub 2017 Jan 9. Brain Connect. 2017. PMID: 27917679 Review. - Neuroaging through the Lens of the Resting State Networks.
Cieri F, Esposito R. Cieri F, et al. Biomed Res Int. 2018 Jan 15;2018:5080981. doi: 10.1155/2018/5080981. eCollection 2018. Biomed Res Int. 2018. PMID: 29568755 Free PMC article. Review.
Cited by
- Fluctuations in Global Brain Activity Are Associated With Changes in Whole-Brain Connectivity of Functional Networks.
Scheinost D, Tokoglu F, Shen X, Finn ES, Noble S, Papademetris X, Constable RT. Scheinost D, et al. IEEE Trans Biomed Eng. 2016 Dec;63(12):2540-2549. doi: 10.1109/TBME.2016.2600248. Epub 2016 Aug 16. IEEE Trans Biomed Eng. 2016. PMID: 27541328 Free PMC article. - Neuroimaging in Pediatric Patients with Mild Traumatic Brain Injury: Relating the Current 2018 Centers for Disease Control Guideline and the Potential of Advanced Neuroimaging Modalities for Research and Clinical Biomarker Development.
Fong AK, Allen MD, Waltzman D, Sarmiento K, Yeates KO, Suskauer S, Wintermark M, Lindberg DM, Tate DF, Wilde EA, Loewen JL. Fong AK, et al. J Neurotrauma. 2021 Jan 1;38(1):44-52. doi: 10.1089/neu.2020.7100. Epub 2020 Oct 21. J Neurotrauma. 2021. PMID: 32640874 Free PMC article. Review. - Aging, sex and cognitive Theory of Mind: a transcranial direct current stimulation study.
Adenzato M, Manenti R, Gobbi E, Enrici I, Rusich D, Cotelli M. Adenzato M, et al. Sci Rep. 2019 Dec 2;9(1):18064. doi: 10.1038/s41598-019-54469-4. Sci Rep. 2019. PMID: 31792263 Free PMC article. Clinical Trial. - Sustained versus instantaneous connectivity differentiates cognitive functions of processing speed and episodic memory.
King JB, Anderson JS. King JB, et al. Hum Brain Mapp. 2018 Dec;39(12):4949-4961. doi: 10.1002/hbm.24336. Epub 2018 Aug 16. Hum Brain Mapp. 2018. PMID: 30113114 Free PMC article. - Data-Driven Analysis of Functional Connectivity Reveals a Potential Auditory Verbal Hallucination Network.
Scheinost D, Tokoglu F, Hampson M, Hoffman R, Constable RT. Scheinost D, et al. Schizophr Bull. 2019 Mar 7;45(2):415-424. doi: 10.1093/schbul/sby039. Schizophr Bull. 2019. PMID: 29660081 Free PMC article.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan, A , Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:2. - PMC - PubMed
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang H.Y, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739. - PMC - PubMed
- Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RW, Théberge J, Williamson P (2008): Default mode network connectivity: Effects of age, sex, and analytic approach. Neuroreport 19:887–891. - PubMed
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical