Dendritic cell metabolism - PubMed (original) (raw)
Review
Dendritic cell metabolism
Edward J Pearce et al. Nat Rev Immunol. 2015 Jan.
Abstract
The past 15 years have seen enormous advances in our understanding of the receptor and signalling systems that allow dendritic cells (DCs) to respond to pathogens or other danger signals and initiate innate and adaptive immune responses. We are now beginning to appreciate that many of these pathways not only stimulate changes in the expression of genes that control DC immune functions, but also affect metabolic pathways, thereby integrating the cellular requirements of the activation process. In this Review, we focus on this relatively new area of research and attempt to describe an integrated view of DC immunometabolism.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
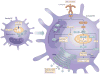
Figure 1. Changes in dendritic cell metabolism through development, quiescence and activation
The development of dendritic cells (DCs) from progenitor cells is associated with mitochondrial biogenesis, which is driven by peroxisome proliferator-activated receptor-γ (PPARγ) co-activator 1α (PGC1α) and promoted by PPARγ, mammalian target of rapamycin (mTOR) and MYC. Differentiated DCs populate their niches as immature DCs. Immature DCs use fatty acid oxidation as a core metabolic process. Activation of DCs by Toll-like receptor (TLR) agonists leads to a rapid increase in flux through glycolysis and the associated pentose phosphate pathway, with an accompanying increase in spare respiratory capacity and fatty acid synthesis. These metabolic changes are initiated by a pathway downstream of TLRs that involves AKT, TANK-binding kinase 1 (TBK1), inhibitor of nuclear factor-κB kinase subunit-ε (IKKε) and hexokinase 2 (HK2), and they are crucial for DC activation. After being activated, DCs remain glycolytic. This process is essential for continued DC survival and is controlled by mTOR and hypoxia-inducible factor 1α (HIF1α). CDP, committed DC progenitor; OXPHOS, oxidative phosphorylation.
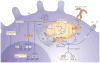
Figure 2. Toll-like receptor signalling integrates endoplasmic reticulum stress and changes in metabolism to support activation
In resting dendritic cells (DCs), fatty acid oxidation is engaged and the endoplasmic reticulum (ER) is relatively constrained. Following exposure to agonists of pattern recognition receptors (PRRs), signalling pathways are activated that lead to the expression of a broad array of nuclear factor-κB (NF-κB)- and interferon-regulatory factor (IRF)-responsive genes. This may lead to ER stress and the activation of the unfolded protein response (UPR) as the cells attempt to accommodate the production of a large set of proteins destined for secretion. A downstream effector of the UPR is X-box-binding protein 1 (XBP1), which transcriptionally activates the genes encoding enzymes for fatty acid synthesis; the UPR seems to be constitutively active in DCs. For Toll-like receptors (TLRs), and potentially other PRRs, this is coupled with activation of AKT, TANK-binding kinase 1 (TBK1), inhibitor of NF-κB kinase subunit-ε (IKKε) and hexokinase 2 (HK2), which promotes increased flux through the glycolysis pathway with resultant increases in citrate export for fatty acid synthesis. This is supported by the coincident increase in activity of the pentose phosphate pathway (PPP), which facilitates the production of NADPH, a crucial cofactor for fatty acid synthesis. Synthesis of new fatty acids allows expansion of the ER, which is likely to relieve ER stress and lead to the production and secretion of effector molecules that are central to DC activation. Thick arrow indicates that fatty acid oxidation is the primary metabolic signature of resting DCs. TCA, tricarboxylic acid.
Figure 3. Mitochondria are foci for the integration of metabolism and innate responses
The mitochondria-associated membranes (MAMs) are areas of close interaction between mitochondria and the endoplasmic reticulum (ER). The MAMs are the sites at which mitochondrial antiviral signalling protein (MAVS) and the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome localize and they serve as a Ca2+ store to maintain mitochondrial calcium concentrations. MAVS interacts with retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) that have sensed viral RNA. The MAVS–RLR complex is then able to initiate signalling to induce expression of cytokines and theoretically promote increased glycolytic flux to support dendritic cell (DC) activation and type I interferon (IFN) responses (indicated by the dashed arrows). MAVS–RLR signalling is dependent on active mitochondria with a high mitochondrial membrane potential (Δψm) and reciprocally serves to promote mitochondrial expansion. NLRP3 is sensitive to reactive oxygen species (ROS) that are produced by the electron transport chain (ETC) and also senses ATP-driven decreases in intracellular potassium concentrations. Once activated, NLRP3 activates caspase 1, which is able to cleave and thereby activate the pro-forms of interleukin-1β (IL-1β) and IL-18. Increased ROS production can be promoted by Toll-like receptor 1 (TLR1), TLR2 and TLR4 signalling through the activation of tumour necrosis factor receptor-associated factor 6 (TRAF6), which relocates to the mitochondria. There, in conjunction with evolutionarily conserved signalling intermediate in Toll pathway (ECSIT), TRAF6 promotes ROS production by the ETC. IKKε, inhibitor of nuclear factor-κB kinase subunit-ε; IRF, IFN-regulatory factor; NF-κB, nuclear factor-κB; P2X7, P2X purinoceptor 7; TBK1, TANK-binding kinase 1.
Similar articles
- Metabolic Control of Dendritic Cell Functions: Digesting Information.
Wculek SK, Khouili SC, Priego E, Heras-Murillo I, Sancho D. Wculek SK, et al. Front Immunol. 2019 Apr 25;10:775. doi: 10.3389/fimmu.2019.00775. eCollection 2019. Front Immunol. 2019. PMID: 31073300 Free PMC article. Review. - Immunometabolism governs dendritic cell and macrophage function.
O'Neill LA, Pearce EJ. O'Neill LA, et al. J Exp Med. 2016 Jan 11;213(1):15-23. doi: 10.1084/jem.20151570. Epub 2015 Dec 22. J Exp Med. 2016. PMID: 26694970 Free PMC article. Review. - Self, non-self, and danger: a complementary view.
Köhl J. Köhl J. Adv Exp Med Biol. 2006;586:71-94. doi: 10.1007/0-387-34134-X_6. Adv Exp Med Biol. 2006. PMID: 16893066 Review. - Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity.
Nace G, Evankovich J, Eid R, Tsung A. Nace G, et al. J Innate Immun. 2012;4(1):6-15. doi: 10.1159/000334245. Epub 2011 Nov 11. J Innate Immun. 2012. PMID: 22086146 Review. - Insights how monocytes and dendritic cells contribute and regulate immune defense against microbial pathogens.
Bieber K, Autenrieth SE. Bieber K, et al. Immunobiology. 2015 Feb;220(2):215-26. doi: 10.1016/j.imbio.2014.10.025. Epub 2014 Oct 31. Immunobiology. 2015. PMID: 25468558 Review.
Cited by
- Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension.
Stenmark KR, Tuder RM, El Kasmi KC. Stenmark KR, et al. J Appl Physiol (1985). 2015 Nov 15;119(10):1164-72. doi: 10.1152/japplphysiol.00283.2015. Epub 2015 Apr 30. J Appl Physiol (1985). 2015. PMID: 25930027 Free PMC article. Review. - Rheumatoid arthritis synovial microenvironment induces metabolic and functional adaptations in dendritic cells.
Canavan M, Marzaioli V, McGarry T, Bhargava V, Nagpal S, Veale DJ, Fearon U. Canavan M, et al. Clin Exp Immunol. 2020 Nov;202(2):226-238. doi: 10.1111/cei.13479. Epub 2020 Jul 15. Clin Exp Immunol. 2020. PMID: 32557565 Free PMC article. Clinical Trial. - Dendritic cells are stressed out in tumor.
Maj T, Zou W. Maj T, et al. Cell Res. 2015 Sep;25(9):989-90. doi: 10.1038/cr.2015.93. Epub 2015 Jul 31. Cell Res. 2015. PMID: 26227962 Free PMC article. - Systemic Immunometabolism: Challenges and Opportunities.
Lercher A, Baazim H, Bergthaler A. Lercher A, et al. Immunity. 2020 Sep 15;53(3):496-509. doi: 10.1016/j.immuni.2020.08.012. Immunity. 2020. PMID: 32937151 Free PMC article. Review. - Autophagic protein ATG5 controls antiviral immunity via glycolytic reprogramming of dendritic cells against respiratory syncytial virus infection.
Oh DS, Park JH, Jung HE, Kim HJ, Lee HK. Oh DS, et al. Autophagy. 2021 Sep;17(9):2111-2127. doi: 10.1080/15548627.2020.1812218. Epub 2020 Aug 28. Autophagy. 2021. PMID: 32816604 Free PMC article.
References
- Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135. - PubMed
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. - PubMed
- Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–664. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous